Abstract
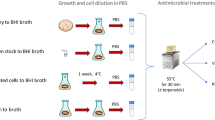
The objective of this study was to explore the recovery of culturability of viable but nonculturable (VBNC) Vibrio parahaemolyticus after temperature upshift and to determine whether regrowth or resuscitation occurred. A clinical strain of V. parahaemolyticus Vp5 was rendered VBNC by exposure to artificial seawater (ASW) at 4°C. Aliquots of the ASW suspension of cells (0.1, 1 and 10 ml) were subjected to increased temperatures of 20°C and 37°C. Culturability of the cells in the aliquots was monitored for colony formation on a rich medium and changes in morphology were measured by scanning (SEM) and transmission (TEM) electron microscopy. Samples of VBNC cells were fixed and examined by SEM, revealing a heterogeneous population comprising small cells and larger, flattened cells. Forty-eight hours after temperature upshift to 20°C or 37°C, both elongation and division by binary fission of the cells were observed, employing SEM and TEM, but only in the 10-ml aliquots. The results suggest that a portion of VBNC cells is able to undergo cell division. It is concluded that a portion of VBNC cells of V. parahaemolyticus subjected to cold temperatures remain viable. After temperature upshift, regrowth of those cells, rather than resuscitation of all bacteria of the initial inoculum, appears to be responsible for recovery of culturability of VBNC cells of V. parahaemolyticus. Nutrient in filtrates of VBNC cells is hypothesized to allow growth of the temperature-responsive cells, with cell division occurring via binary fission, but also including an atypical, asymmetric cell division.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bates TC, Oliver JD . (2004). The viable but nonculturable state of Kanagawa positive and negative strains of Vibrio parahaemolyticus. J Microbiol 42: 74–79.
Bjedov I, Tenaillon O, Gerard B, Souza V, Denamur E, Radman M et al. (2003). Stress-induced mutagenesis in bacteria. Science 300: 1404–1409.
Bogosian G, Aardema ND, Bourneuf EV, Morris PJ, O’Neil JP . (2000). Recovery of hydrogen peroxide-sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J Bacteriol 182: 5070–5075.
Chowdhury MAR, Yamanaka H, Miyoshi SI, Shinoda S . (1990). Ecology and seasonal distribution of Vibrio parahaemolyticus in aquatic environments of temperate region. FEMS Microbiol Ecol 74: 1–10.
Coutard F, Pommepuy M, Loaec S, Hervio-Heath D . (2005). mRNA detection by reverse transcription-PCR for monitoring viability and potential virulence in a pathogenic strain of Vibrio parahaemolyticus in viable but nonculturable state. J Appl Microbiol 98: 951–961.
Firth JR, Diaper JP, Edwards C . (1994). Survival and viability of Vibrio vulnificus in seawater monitored by flow cytometry. Lett Appl Microbiol 18: 268–271.
Fischer-Le Saux M, Hervio-Heath D, Loaec S, Colwell RR, Pommepuy M . (2002). Detection of cytotoxin–hemolysin mRNA in nonculturable populations of environmental and clinical Vibrio vulnificus strains in artificial seawater. Appl Environ Microbiol 68: 5641–5646.
Gauthier MJ . (2000). Environmental parameters associated with the viable but nonculturable state. In: Colwell RR (ed). Nonculturable Microorganisms in the Environment. ASM Press: Washington, pp 87–112.
Jiang X, Chai TJ . (1996). Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl Environ Microbiol 62: 1300–1305.
Kogure K, Simidu U, Taga N . (1979). A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol 25: 415–420.
Lleo MM, Pierobon S, Tafi MC, Signoretto C, Canepari P . (2000). mRNA detection by reverse transcription-PCR for monitoring viability over time in an Enterococcus faecalis viable but nonculturable population maintained in a laboratory microcosm. Appl Environ Microbiol 66: 4564–4567.
Matic I, Taddei F, Radman M . (2004). Survival versus maintenance of genetic stability: a conflict of priorities during stress. Res Microbiol 155: 337–341.
Mizunoe Y, Wai SN, Ishikawa T, Takade A, Yoshida S . (2000). Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol Lett 186: 115–120.
Nilsson L, Oliver JD, Kjelleberg S . (1991). Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol 173: 5054–5059.
Oliver JD . (1993). Formation of viable but nonculturable cells. In: Kjelleberg S (ed). Starvation in Bacteria. Plenum Press: New York, pp 239–272.
Oliver JD, Nilsson L, Kjelleberg S . (1991). Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol 57: 2640–2644.
Oliver JD, Hite F, McDougald D, Andon N, Simpson L . (1995). Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol 61: 2624–2630.
Porter KG, Feig YS . (1980). The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25: 943–948.
Postgate JR, Hunter JR . (1962). The survival of starved bacteria. J Gen Microbiol 29: 233–263.
Rahman I, Shahamat M, Kirchman PA, Russek-Cohen E, Colwell RR . (1994). Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol 60: 3573–3578.
Ravel J, Knight IT, Monahan CE, Hill RT, Colwell RR . (1995). Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology 141: 377–383.
Rodriguez GG, Phipps D, Ishiguro K, Ridgway HF . (1992). Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol 58: 1801–1808.
Roszak DB, Grimes DJ, Colwell RR . (1984). Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol 30: 334–338.
Sheridan GE, Masters CI, Shallcross JA, MacKey BM . (1998). Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 64: 1313–1318.
Smith B, Oliver JD . (2006). In situ and in vitro gene expression by Vibrio vulnificus during entry into, persistence within, and resuscitation from the viable but nonculturable state. Appl Environ Microbiol 72: 1445–1451.
Weichart D, McDougald D, Jacobs D, Kjelleberg S . (1997). In situ analysis of nucleic acids in cold-induced nonculturable Vibrio vulnificus. Appl Environ Microbiol 63: 2754–2758.
Weichart D, Oliver JD, Kjelleberg S . (1992). Low temperature induced non-culturability and killing of Vibrio vulnificus. FEMS Microbiol Lett 100: 205–210.
Whitesides MD, Oliver JD . (1997). Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol 63: 1002–1005.
Wong HC, Wang P, Chen SY, Chiu SW . (2004). Resuscitation of viable but non-culturable Vibrio parahaemolyticus in a minimum salt medium. FEMS Microbiol Lett 233: 269–275.
Xu HS, Roberts NC, Singleton FL, Attwell RW, Grimes DJ, Colwell RR . (1982). Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbiol Ecol 8: 313–323.
Yaron S, Matthews KR . (2002). A reverse transcriptase-polymerase chain reaction assay for detection of viable Escherichia coli O157:H7: investigation of specific target genes. J Appl Microbiol 92: 633–640.
Zimmermann R, Iturriaga R, Becker-Birck J . (1978). Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol 36: 926–935.
Acknowledgements
This project was supported by a grant from the French Research Institute for Exploitation of the Sea (IFREMER) and the Région Pays de la Loire. We thank Mrs Françoise S Le Guyader (IFREMER, Laboratoire de Microbiologie, Nantes, France) for critical reading of the paper and Mr Jacques Le Pendu (INSERM U601, Institut de Biologie, Nantes, France) for his advice on scientific aspects of the project. We also thank Mr Jean-Michel Fournier (Institut Pasteur, Unité du Choléra et des Vibrions, Centre National de Référence des Vibrions et du Choléra, Paris, France) for providing the clinical strain (CNRVC 970136) of V. parahaemolyticus used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coutard, F., Crassous, P., Droguet, M. et al. Recovery in culture of viable but nonculturable Vibrio parahaemolyticus: regrowth or resuscitation?. ISME J 1, 111–120 (2007). https://doi.org/10.1038/ismej.2007.1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2007.1
Keywords
This article is cited by
-
Effect of physicochemical and microbiological factors on the development of viable but non-culturable and resuscitation states of Vibrio cholerae
Archives of Microbiology (2024)
-
Response mechanism of Vibrio parahaemolyticus at high pressure revealed by transcriptomic analysis
Applied Microbiology and Biotechnology (2022)
-
Optimization of resuscitation-promoting broths for the revival of Vibrio parahaemolyticus from a viable but nonculturable state
Food Science and Biotechnology (2021)
-
Survival strategies of Escherichia coli and Vibrio spp.: contribution of the viable but nonculturable phenotype to their stress-resistance and persistence in adverse environments
World Journal of Microbiology and Biotechnology (2017)
-
Changes in the Vibrio harveyi Cell Envelope Subproteome During Permanence in Cold Seawater
Microbial Ecology (2016)