Abstract
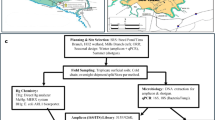
DNA was extracted from different depth soils (0–5, 45–55 and 90–100 cm below surface) sampled at Lower East Fork Poplar Creek floodplain (LEFPCF), Oak Ridge (TN, USA). The presence of merA genes, encoding the mercuric reductase, the key enzyme in detoxification of mercury in bacteria, was examined by PCR targeting Actinobacteria, Firmicutes or β/γ-Proteobacteria. β/γ-Proteobacteria merA genes were successfully amplified from all soils, whereas Actinobacteria were amplified only from surface soil. merA clone libraries were constructed and sequenced. β/γ-Proteobacteria sequences revealed high diversity in all soils, but limited vertical similarity. Less than 20% of the operational taxonomic units (OTU) (DNA sequences ⩾95% identical) were shared between the different soils. Only one of the 62 OTU was ⩾95% identical to a GenBank sequence, highlighting that cultivated bacteria are not representative of what is found in nature. Fewer merA sequences were obtained from the Actinobacteria, but these were also diverse, and all were different from GenBank sequences. A single clone was most closely related to merA of α-Proteobacteria. An alignment of putative merA genes of genome sequenced mainly marine α-Proteobacteria was used for design of merA primers. PCR amplification of soil α-Proteobacteria isolates and sequencing revealed that they were very different from the genome-sequenced bacteria (only 62%–66% identical at the amino-acid level), although internally similar. In light of the high functional diversity of mercury resistance genes and the limited vertical distribution of shared OTU, we discuss the role of horizontal gene transfer as a mechanism of bacterial adaptation to mercury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Abulencia CB, Wyborski DL, Garcia JA, Podar M, Chen W, Chang SH et al. (2006). Environmental whole-genome amplification to access microbial populations in contaminated sediments. Appl Environ Microbiol 72: 3291–3301.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402.
Barkay T, Miller SM, Summers AO . (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev 27: 355–384.
Barnett MO, Harris LA, Turner RR, Henson TJ, Melton RE, Stevenson RJ . (1995). Characterization of mercury species in contaminated floodplain soils. Water Air Soil Pollution 80: 1105–1108.
Burger J, Campbell KR . (2004). Species differences in contaminants in fish on and adjacent to the Oak Ridge Reservation, Tennessee. Environ Res 96: 145–155.
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG et al. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500.
Davis KER, Joseph SJ, Janssen PH . (2005). Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl Environ Microbiol 71: 826–834.
Distefano MD, Moore MJ, Walsh CT . (1990). Active site of mercuric reductase resides at the subunit interface and requires Cys135 and Cys140 from one subunit and Cys558 and Cys559 from the adjacent subunit: evidence from in vivo and in vitro heterodimer formation. Biochemistry 29: 2703–2713.
Ellis RJ, Morgan P, Weightman AJ, Fry JC . (2003). Cultivation-dependent and -independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl Environ Microbiol 69: 3223–3230.
Eto K . (2000). Minamata disease. Neuropathology 20 (Suppl): S14–S19.
Gans J, Wolinsky M, Dunbar J . (2005). Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309: 1387–1390.
Griffin HG, Foster TJ, Silver S, Misra TK . (1987). Cloning and DNA-sequence of the mercuric-resistance and organomercurial-resistance determinants of plasmid pDU1358. Proc Natl Acad Sci USA 84: 3112–3116.
Han FX, Su Y, Monts DL, Waggoner CA, Plodinec MJ . (2006). Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci Total Environ 368: 753–768.
Hartmann M, Widmer F . (2006). Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl Environ Micorbiol 72: 7804–7812.
Huang CC, Narita M, Yamagata T, Endo G . (1999). Identification of three merB genes and characterization of a broad-spectrum mercury resistance module encoded by a class II transposon of Bacillus megaterium strain MB1. Gene 239: 361–366.
Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M . (2002). Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68: 2391–2396.
Janssen PH . (2006). Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72: 1719–1728.
Laddaga RA, Chu L, Misra TK, Silver S . (1987). Nucleotide sequence and expression of the mercurial-resistance operon from Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci USA 84: 5106–5110.
Ledwidge R, Patel B, Dong A, Fiedler D, Falkowski M, Zelikova J et al. (2005). NmerA, the metal binding domain of mercuric ion reductase, removes Hg2+ from proteins, delivers it to the catalytic core, and protects cells under glutathione-depleted conditions. Biochemistry 44: 11402–11416.
Lipthay JRd, Rasmussen LD, Oregaard G, Simonsen K, Bahl MI, Kroer N et al. (2007). The adaptive potential of subsurface microbial communities to mercury. FEMS Microbiol Ecol (submitted).
Lowe M, Madsen EL, Schindler K, Smith C, Emrich S, Robb F et al. (2002). Geochemistry and microbial diversity of a trichlorethane-contaminated superfund site undergoing intrinsic in situ reductive dechlorination. FEMS Microbiol Ecol 40: 123–134.
Mongodin EF, Nelson KE, Daugherty S, Deboy RT, Wister J, Khouri H et al. (2005). The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc Natl Acad Sci USA 102: 18147–18152.
Moore MJ, Walsh CT . (1989). Mutagenesis of the N- and C-terminal cysteine pairs of Tn501 mercuric ion reductase: consequences for bacterial detoxification of mercurials. Biochemistry 28: 1183–1194.
Moore MJ, Miller SM, Walsh CT . (1992). C-terminal cysteines of Tn501 mercuric ion reductase. Biochemistry 31: 1677–1685.
Muller AK, Rasmussen LD, Sørensen SJ . (2001a). Adaptation of the bacterial community to mercury contamination. FEMS Microbiol Lett 204: 49–53.
Muller AK, Westergaard K, Christensen S, Sørensen SJ . (2001b). The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiol Ecol 36: 11–19.
Ni Chadhain SM, Schaefer JK, Crane S, Zylstra GJ, Barkay T . (2006). Analysis of mercuric reductase (merA) gene diversity in an anaerobic mercury-contaminated sediment enrichment. Environ Microbiol 8: 1746–1752.
Nies DH . (1999). Microbial heavy-metal resistance. Appl Microbiol and Biotechnol 51: 730–750.
North NN, Dollhopf SL, Petrie L, Istok JD, Balkwill DL, Kostka JE . (2004). Change in bacterial community structure during in situ biostimulation of subsurface sediment cocontaminated with uranium and nitrate. Appl Environ Microbiol 70: 4911–4920.
Nriagu JO, Pacyna JM . (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333: 134–139.
Oregaard G, Sørensen SJ . (2007). Cultivation of mercury resistant bacteria from mercury contaminated soil (Oak Ridge, Tennessee, USA). FEMS Microbiol Ecol (submitted).
Osborn AM, Bruce KD, Strike P, Ritchie DA . (1997). Distribution diversity evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol Rev 19: 239–262.
Pinhassi J, Bowman JP, Nedashkovskaya OI, Lekunberri I, Gomez-Consarnau L, Pedros-Alio C . (2006). Leeuwenhoekiella blandensis sp. nov., a genome-sequenced marine member of the family Flavobacteriaceae. Int J Syst Evol Microbiol 56: 1489–1493.
Rasmussen L, Sørensen SJ . (2001). Effects of mercury contamination on the culturable heterotrophic, functional and genetic diversity of the bacterial community in soil. FEMS Microbiol Ecol 36: 1–9.
Rasmussen LD, Sørensen SJ . (1998). The effect of longterm exposure to mercury on the bacterial community in marine sediment. Curr Microbiol 36: 291–297.
Revis NW, Osborne TR, Holdsworth G, Hadden C . (1989). Distribution of mercury species in soil from a mercury-contaminated site. Water Air Soil Pollution 45: 105–113.
Schaefer JK, Yagi J, Reinfelder JR, Cardona T, Ellickson KM, Tel-Or S et al. (2004). Role of the bacterial organomercury lyase (MerB) in controlling methylmercury accumulation in mercury-contaminated natural waters. Environ Sci Technol 38: 4304–4311.
Schaffer AA, Aravind L, Madden TL, Shavirin S, Spouge JL, Wolf YI et al. (2001). Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res 29: 2994–3005.
Schelert J, Dixit V, Hoang V, Simbahan J, Drozda M, Blum P . (2004). Occurrence and characterization of mercury resistance in the hyperthermophilic archaeon Sulfolobus solfataricus by use of gene disruption. J Bacteriol 186: 427–437.
Schiering N, Kabsch W, Moore MJ, Distefano MD, Walsh CT, Pai EF . (1991). Structure of the detoxification catalyst mercuric ion reductase from Bacillus sp. strain RC607. Nature 352: 168–172.
Schloss PD, Handelsman J . (2005). Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71: 1501–1506.
Schneiker S, Keller M, Droge M, Lanka E, Puhler A, Selbitschka W . (2001). The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res 29: 5169–5181.
Schuster PF, Krabbenhoft DP, Naftz DL, Cecil LD, Olson ML, Dewild JF et al. (2002). Atmospheric mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environ Sci Technol 36: 2302–2310.
Silver S, Phung LT . (1996). Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50: 753–789.
Sørensen SJ, Bailey M, Hansen LH, Kroer N, Wuertz S . (2005). Studying plasmid horizontal transfer in situ: a critical review. Nat Rev Microbiol 3: 700–710.
Sørensen SJ, de Lipthay JR, Müller AK, Barkay T, Hansen LH, Rasmussen LD . (2002). Molecular methods for assessing and manipulating the diversity of microbial populations and processes. In: Burns RG, Dick RP (eds). Enzymes in the Environment. Marcel Dekker Inc.: New York, pp 363–390.
Stevens RT, Ashwood TL, Sleeman JM . (1997). Mercury in hair of Muskrats (Ondatra zibethicus) and Mink (Mustela vison) from the US Department of Energy Oak Ridge Reservation. Bull Environ Contaminat Toxicol 58: 720–725.
Tomiyasu T, Matsuyama A, Eguchi T, Fuchigami Y, Oki K, Horvat M et al. (2006). Spatial variations of mercury in sediment of Minamata Bay, Japan. Sci Total Environ 368: 283–290.
Top EM, Springae Dl, Boon N . (2002). Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol 42: 199–208.
Vetriani C, Chew YS, Miller SM, Yagi J, Coombs J, Lutz RA et al. (2005). Mercury adaptation among bacteria from a deep-sea hydrothermal vent. Appl Environ Microbiol 71: 220–226.
Wang Y, Mahler I, Levinson HS, Halvorson HO . (1987). Cloning and expression in Escherichia coli of chromosomal mercury resistance genes from a Bacillus sp. J Bacteriol 169: 4848–4851.
Wernersson R, Pedersen AG . (2003). RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res 31: 3537–3539.
Zhou J, Xia B, Huang H, Palumbo AV, Tiedje JM . (2004). Microbial diversity and heterogeneity in sandy subsurface soils. Appl Environ Microbiol 70: 1723–1734.
Acknowledgements
This research was supported by the Natural and Accelerated Bioremediation Research (NABIR) program, Biological and Environmental Research (BER), US Department of Energy, The Villum Kann Rasmussens foundation, and The Danish Natural Science Research Council. We thank David Watson (NABIR Field Research Center, Oak Ridge National Laboratory, TN, USA) for providing soil samples. The skilled technical assistance of Karin P. Vestberg is highly acknowledged. Kristoffer Simonsen is acknowledged for providing plasmids P1, P2, P3 and P4 and assisting in PCR amplification and sequencing of the merA genes of these plasmids. The Leeuwenhoekiella blandensis isolate referred to in the discussion was a kind gift of Dr Jarone Pinhassi at Kalmar University, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oregaard, G., Sørensen, S. High diversity of bacterial mercuric reductase genes from surface and sub-surface floodplain soil (Oak Ridge, USA). ISME J 1, 453–467 (2007). https://doi.org/10.1038/ismej.2007.56
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2007.56
Keywords
This article is cited by
-
Diverse hydrocarbon degradation genes, heavy metal resistome, and microbiome of a fluorene-enriched animal-charcoal polluted soil
Folia Microbiologica (2024)
-
Response of bacterial communities to mining activity in the alpine area of the Tianshan Mountain region, China
Environmental Science and Pollution Research (2021)
-
Unravelling the antibiotic and heavy metal resistome of a chronically polluted soil
3 Biotech (2020)
-
Interactions between Hg and soil microbes: microbial diversity and mechanisms, with an emphasis on fungal processes
Applied Microbiology and Biotechnology (2020)
-
Mercury bioremediation by mercury resistance transposon-mediated in situ molecular breeding
Applied Microbiology and Biotechnology (2018)