Abstract
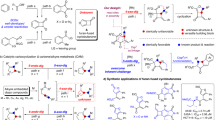
The [2+2]cycloaddition of chlorosulfonyl isocyanate to vinyl and (Z)-propenyl ethers derived from the 2-O-sulfonylated (R)- and (S)-1-(furyl-2′)-1,2-ethanediols furnished the 4-alkoxy-azetidin-2-ones with a good to moderate stereoselectivity. The intramolecular alkylation of the β-lactam nitrogen atom led to the corresponding 3-(furyl-2′)- and 6-methyl-3-(furyl-2′)-clavams. The transformation of the furyl residue into an alkoxycarbonyl group led to clavams related to the natural compounds. The synthesized clavams exhibited moderate inhibitory activities against DD-peptidase 64-575 and β-lactamase (penase) as well as antifungal activities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
a) Brown AG, Butlerworth D, Cole M, Hanscomb G, Hood JD, Reading C, Rolinson GN . Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot 29: 668–669 ( 1976)
b) Brown AG, Corbett DF, Goodacre J, Harbridge JB, Howarth TT, Ponsford RJ, Stirling I, King TI . Clavulanic acid and its derivatives. Structure elucidation of clavulanic acid and the preparation of dihydroclavulanic acid, isoclavulanic acid, esters and related oxidation products. J Chem Soc, Perkin Trans 1: 635–650 ( 1984)
Brown DB, Evans JR, Fletton RA . Structures of three novel β-lactams isolated from Streptomyces clavuligerus. J Chem Soc, Chem Commun 282–283 ( 1979)
Wanning M, Zahner H, Krone B, Zeeck A . Ein neues antifungisches β-lactam-antibioticum der clavam-reihe. Tetrahedron Lett 22: 2539–2540 ( 1981)
a) Pruess DL, Kellett M . Ro 22-5417, a new clavam antibiotic from Streptomyces clavuligerus. I. Discovery and biological activity. J Antibiot 36: 208–212 ( 1983)
b) Evans RH Jr, Ax H, Jacoby A, Williams TH, Jenkis E, Scanneli JP . Ro 22-5417, A new clawam antibiotic from Streptomyces clavuligerus. II. Fermentation, isolation and structure. J Antibiot 36: 213–216 ( 1983)
a) King HD, Langhärig J, Sanglier JJ . Clavamycins, new clavam antibiotics from two variants of Streptomyces hygroscopicus. I. Taxonomy of the producing organisms, fermentation, and biological activities. J Antibiot 39: 510–515 ( 1986)
b) Naegeri HV, Loosli H-R, Nussbaumer A . Clavamycins, new clavam antibiotics from two variants of Streptomyces hygroscopicus. II. Isolation and structures of clavamycins A, B and C from Streptomyces hygroscopicus NRRL 15846, and of clavamycins D, E anf F from Streptomyces hygroscopius NRRL 15879. J Antibiot 39: 516–524 ( 1986)
Veinberg G, Vorona M, Shestakova I, Kanepe I, Lukevics E . Design of β-lactams with mechanism based nonantibacterial activities. Curr Med Chem 10: 1741–1757 ( 2003)
Clauss K, Grimm D, Prossel G . β-Lactame mit über Heteroatome gebundenen Substituenten. Liebigs Ann Chem 539–560 ( 1974)
De Bernardo S, Tengi JP, Sasso GJ, Weigele M . Clavalanine (Ro 22-5417), a new clavam antibiotic from Streptomyces clavuligerus. 4. A Stereorational synthesis. J Org Chem 50: 3457–3462 ( 1985)
Müller JC, Toome V, Pruess DL, Blount JF, Weigele M . Ro 22-5417, A new clavam antibiotic from Streptomyces clavuligerus. III. Absolute stereochemistry. J Antibiot 36: 217–225 ( 1983)
Hoppe D, Hilpert T . Enantioselective total synthesis of the fungicide β-lactam antibiotic (−)-(2S,5S)-2-(2-hydroxyethyl)clavam and its (+)-(2S,5R)-epimer. Tetrahedron 43: 2467–2474 ( 1987)
Kaluza Z, Furman B, Krajewski P, Chmielewski M . Strategies for the formation of 1-dethia-1-oxa-cephams. Tetrahedron 56: 5553–5562 ( 2000)
a) Chmielewski M, Kaluża Z, Furman B . Stereocontrolled synthesis of 1-oxabicyclic β-lactam antibiotics via [2+2]cycloaddition of isocyanates to sugar vinyl ethers. J Chem Soc, Chem Commun 2689–2696 ( 1996)
b) Furman B, Kaluża Z, Lysek R, Chmielewski M . [2+2]Cycloaddition of chlorosulfonyl isocyanate to chiral vinyl ethers. Polish J Chem 73: 43–54 ( 1999)
Borsuk K, Suwin´ska K, Chmielewski M . Stereocontrolled formation of cephams from 1,3-O-ethylidene-L-erythritol. Tetrahedron: Asymmetry 12: 979–981 ( 2001)
Borsuk K, Kazimierski A, Solecka J, Urban´czyk-Lipkowska Z, Chmielewski M . Stereocontrolled formation of oxacephams from carbohydrates. Carbohydr Res 337: 2005–2015 ( 2002)
Kaluża Z, Furman B, Patel M, Chmielewski M . Asymmetric induction in [2+2]cycloaddition of chlorosulfonyl isocyanate to 1,2-O-isopropylidene-3-O-vinyl-glycofuranoses. Tetrahedron: Asymmetry 5: 2179–2186 ( 1994)
Kaluża Z, Furman B, Chmielewski M . Asymmetric induction in [2+2]cycloaddition of chlorosulfonyl isocyanate to 1,2-O-isopropylidene-5-O-vinyl-D-glycofuranoses. Tetrahedron: Asymmetry 6: 1719–1730 ( 1995)
Neuß O, Furman B, Kaluża Z, Chmielewski M . Synthesis of dioxolanylclavam from tartaric acid. Heterocycles 45: 265–270 ( 1997)
a) Cierpucha M, Solecka J, Frelek J, Szczukiewicz P, Chmielewski M . Synthesis, biological and chiroptical activity of 3-phenyl-clavams. Bioorg Med Chem 12: 405–416 ( 2004)
b) Chmielewski M, Cierpucha M, Kowalska P, Kwit M, Frelek J . Structure-chiroptical properties relationship in clavams: an experimental and theoretical study. Chirality, in press
a) Gonzales F, Lesage S, Perlin AS . Catalysis by mercuric ion of reactions of glycals with water. Carbohydr Res 42: 267–274 ( 1975)
b) Hayashi M, Kawabata H, Yamada K . Metal-catalyzed transformation of D-glucal to optically active furan diol. J Chem Soc, Chem Commun 965–966 ( 1999)
c) Agarwal A, Rani S, Vankar YD . Protic acid (HClO4 supported on silica gel)-mediated synthesis of 2,3-unsaturated-O-glucosides and a chiral furan diol from 2,3-glycals. J Org Chem 69: 6137–6140 ( 2004)
Saeed M, Ilg T, Schick M, Abbas M, Voelter W . Total synthesis and anti-leishmanial activity of R-(−)-argentilactone. Tetrahedron Lett 42: 7401–7403 ( 2001)
Prosser TJ . The Rearrangement of allyl ethers to propenyl ethers. J Am Chem Soc 83: 1701–1704 ( 1961)
Watanabe WH, Conlon LE . Homogeneous metal salt catalysis in organic reactions. I. The preparation of vinyl ethers by vinyl transetherification. J Am Chem Soc 79: 2828–2833 ( 1957)
Okimoto Y, Sakaguchi S, Ishii Y . Development of a highly efficient catalytic method for synthesis of vinyl ethers. J Am Chem Soc 124: 1590–1591 ( 2002)
Furman B, Kaluża Z, Chmielewski M . An approach to clavams and 1-oxacephams from hydroxy acids. J Org Chem 62: 3135–3139 ( 1997)
Borsuk K, Grzeszczyk B, Szczukiewicz P, Przykorska B, Frelek J, Chmielewski M . Stereoselectivity in formation of oxacephams from 1,3-alkylidene-threitols. Chirality 16: 414–421 ( 2004)
Arribas E, Carreiro C, Valdeolmillos AM . Total synthesis of (±)-clavam-2-carboxylic acid. Tetrahedron Lett 29: 1609–1612 ( 1988)
Neu HC . Structure-activity relationships: biological. In The Chemistry of β-lactams, Ed. M. I. Page, Blackie Academic & Professional, pp. 101–127 ( 1992)
Frére JM, Leyh-Bouille M, Ghuysen JM, Nieto M, Perkins HR . Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol 45: 610–636 ( 1976)
Kurzatkowski W, Solecka J, Filipek J, Kurzatkowski JD, Kurylowicz W . Streptomycetes excreting DD-carboxypeptidases. Appl Microbiol Biotechnol 33: 452–454 ( 1990)
Röhl F, Rabenhorst J, Zähner H . Biological properties and mode of action of clavams. Arch Microbiol 147: 315–320 ( 1987)
Solecka J, Kurzatkowski W . Affinity of exocellular DD-carboxypeptidase/transpeptidase from Saccharopolyspora erythraea PZH TZ-575 to beta-lactam compounds. Med Dos´w Mikrobiol 51: 151–165 ( 1999)
Solecka J, Lysek R, Furman B, Chmielewski M, Kurzatkowski W . Practical use of DD-peptidase 64-575 for assay inhibition activity of natural and synthetic β-lactam compounds. Acta Poloniae Pharmaceutica 60: 115–118 ( 2003)
O'Callaghan CH, Morris A, Kirby SM, Shingler AH . Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother 1: 283–288 ( 1972)
Barry AL, Pfaller MA, Rennie RP, Fuchs PC, Brown SD . Precision and accuracy of fluconazole susceptibility testing by broth microdilution, etest, and disk diffusion methods. Antimicrob Agents Chemother 46: 1781–1784 ( 2002)
Kirkpatrick WR, Turner TM, Fothergill AW, McCarthy DI, Redding SW, Rinaldi MG, Patterson TF . Fluconazole disk diffusion susceptibility testing of Candida species. J Clin Microbiol 36: 3429–3432 ( 1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cierpucha, M., Panfil, I., Danh, T. et al. Synthesis of 3-Substituted-clavams: Antifungal Properties, DD-Peptidase and β-Lactamase Inhibition. J Antibiot 60, 622–632 (2007). https://doi.org/10.1038/ja.2007.80
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/ja.2007.80