Abstract
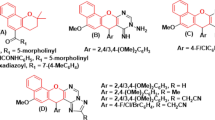
A series of novel 3-O-(3-aryl-propenyl)clarithromycin derivatives were designed, synthesized and evaluated for their in vitro antibacterial activities. Regioselective allylation at 3-OH was efficiently achieved in the presence of 9-oxime ether, compared with 9-keto. Most of the side chains were identified as the 3-O-(3-aryl-Z-prop-1-enyl) group, not the expected 3-O-(3-aryl-E-prop-2-enyl) group. Some derivatives of this series showed improved activities against erythromycin-resistant Staphylococcus aureus and Staphylococcus pneumoniae compared with the reference compound, clarithromycin, but weaker activities against susceptible strains.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kurath, P., Jones, P. H., Egan, R. S. & Perun, T. J. Acid degradation of erythromycin A and erythromycin B. Experientia 27, 362 (1971).
Watanabe, Y., Morimoto, S., Adachi, T., Kashimura, M. & Asaka, T. Chemical modification of erythromycins. 9. Selective methylation the C-6 hydroxyl group of erythromycin A oxime derivatives and preparation of clarithromycin. J. Antibiot. 46, 647–660 (1993).
Plata, D. J. et al. The synthesis of ketolide antibiotic ABT-773 (cethromycin). Tetrahedron 60, 10171–10180 (2004).
Ma, Z. et al. Novel erythromycin derivatives with aryl groups tethered to the C-6 position are potent protein synthesis inhibitors and active against multidrug-resistant respiratory pathogens. J. Med. Chem. 44, 4137–4156 (2001).
Clark, R. F. et al. Synthesis and antibacterial activity of novel 6-O-substituted erythromycin A derivatives. Bioorg. Med. Chem. Lett. 10, 815–819 (2000).
Agouridas, C. et al. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J. Med. Chem. 41, 4080–4100 (1998).
Tanikawa, T. et al. Synthesis and antibacterial activity of acylides (3-O-acyl-erythromycin derivatives): a novel class of macrolide antibiotics. J. Med. Chem. 44, 4027–4030 (2001).
Tang, D. et al. Design, synthesis, and antibacterial activities of novel 3,6-bicyclolide oximes: length optimization and zero carbon linker oximes. Bioorg. Med. Chem. Lett. 18, 5078–5082 (2008).
Elliott, R. L. et al. Anhydrolide macrolides. 1. Synthesis and antibacterial activity of 2,3-anhydro-6-O-methyl 11,12-carbamate erythromycin A analogues. J. Med. Chem. 41, 1651–1659 (1998).
Denis, A. et al. Beta-keto-ester chemistry and ketolides. Synthesis and antibacterial activity of 2-halogeno, 2-methyl and 2,3 enol-ether ketolides. Bioorg. Med. Chem. Lett. 10, 2019–2022 (2000).
Elliott, R. L. et al. Novel 3-deoxy-3-descladinosyl-6-O-methyl erythromycin A analogues. Synthesis and in vitro activity. Bioorg. Med. Chem. Lett. 7, 641–646 (1997).
Misawa, Y., Asaka, T., Kashimura, M., Morimoto, S. & Hatayama, K. 5-O-desosaminylerythronolide derivative EP 682, 038 24 January (1994).
Cheng, H. M. et al. Synthesis and SAR of azalide 3,6-ketal aromatic derivatives as potent gram-positive and gram-negative antibacterial agents. Bioorg. Med. Chem. Lett. 12, 2431–2434 (2002).
Heggelund, A. & Undheim, K. Descladinosyl erythromycin in phosgene-assisted cyclic 3,6-ether formation. Tetrahedron Lett. 49, 5569–5571 (2008).
Douthwaite, S., Hansen, L. H. & Mauwais, P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol. Microbiol. 36, 183–192 (2000).
Furuuchi, T. et al. Design and synthesis of novel leucomycin analogues modified at the C-3 position. Part II: 3-O-(3-Aryl-2-propenyl)leucomycin analogues. Bioorg. Med. Chem. 16, 4401–4418 (2008).
Watanabe, Y. et al. Chemical modification of erythromycins. 12. A facile synthesis of clarithromycin (6-O-methylerythromycin A) via 2′-silylethers of erythromycin A derivatives. J. Antibiot. 46, 1163–1167 (1993).
Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard M7-A7, Seventh Edition, Clinical and Laboratory Standards Institute, Wayne, PA, (2006).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (20602002). We thank Prof. Xiang Hao and Ms. Tong-Ling Liang, Institute of Chemistry Chinese Academy of Sciences, for single X-ray crystal structure determination.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, JH., Wang, YY., Zhu, DY. et al. Design, synthesis and antibacterial activity of a novel alkylide: 3-O-(3-aryl-propenyl)clarithromycin derivatives. J Antibiot 62, 605–611 (2009). https://doi.org/10.1038/ja.2009.89
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2009.89
Keywords
This article is cited by
-
Synthesis and antibacterial activity of 2, 3-dehydro-3-O-(3-aryl-E-prop-2-enyl)-10, 11-anhydroclarithromycin derivatives
The Journal of Antibiotics (2011)