Abstract
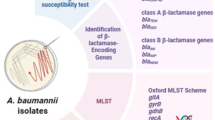
The objective of this work was to investigate correlations between Acinetobacter baumannii isolates from neurosurgical intensive care unit patients and its environment. This is a prospective, observational study. The minimal inhibitory concentrations of antimicrobial agents against 27 clinical and 28 environmental isolates were determined by the agar dilution method. Molecular genotyping was performed by enterobacterial repetitive intergenic consensus PCR (ERIC-PCR), pulsed-field gel electrophoresis (PFGE) and multi-locus sequence typing (MLST). The presence of carbapenemase and metallo-β-lactamase genes were analyzed by specific PCRs and DNA sequencing. From the clinical A. baumannii isolates, 25.9% were found resistant to minocycline, 51.9% to cefoperazone-sulbactam, 59.3% to imipenem and 70% resistant to other antimicrobial agents. Environmental isolates were more sensitive compared with clinical isolates (P<0.05). Twenty-seven clinical isolates comprised three ERIC-PCR genotypes, four major PFGE pulsotypes and five distinct MLST sequence types (STs) (ST208, ST368, ST191, ST195, ST540), all belonging to CC92 with only one locus (gpi) difference among them. Twenty-eight environmental isolates showed more diverse genetic types than clinical isolates and comprised six ERIC-PCR groups, nine PFGE groups and two main STs (ST208, ST229). Four clinical and 15 environmental isolates could not be identified by MLST and were assigned to non-clonal STs. We identified the presence of the blaOXA-23 carbapenemase encoding gene in most of the clinical (21/27) but fewer in the environmental isolates (3/28). The A. baumannii strains isolated from patients were genetically similar to the environmental strains, with CC92 members as the major fraction but with different antibiotic susceptibilities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abbott, I., Cerqueira, G. M., Bhuiyan, S. & Peleg, A. Y. Carbapenem resistance in Acinetobacter baumannii: laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev. Anti Infect. Ther. 11, 395–409 (2013).
Chen, Z. et al. Molecular epidemiology of carbapenem-resistant Acinetobacter spp. from XiangYa Hospital, in Hunan Province, China. J. Basic Microbiol. 53, 121–127 (2003).
Levin, A. S., Gobara, S., Mendes, C. M. F., Cursino, M. R. & Sinto, S. Environmental contamination by multidrug-resistant Acinetobacter baumannii in an intensive care unit. Infect. Control Hosp. Epidemiol. 22, 717–720 (2001).
Dai, X. T. et al. The epidemiology and resistance mechanisms of Acinetobacter baumannii isolates from the respiratory department ICU of a hospital in China. Microb. Drug Resist. 20, 618–622 (2014).
Chang, K. M. et al. Suitable restriction enzyme for standardization of pulsed-field gel electrophoresis protocol and interlaboratory comparison of Acinetobacter baumannii. J. Microbiol. Immunol. Infect. 46, 195–201 (2013).
Cristina, M. L., Spagnolo, A. M. & Cenderello, N. Multidrug-resistant Acinetobacter baumannii outbreak: an investigation of the possible routes of transmission. Public Health 127, 386–391 (2013).
Diancourt, L., Passet, V., Nemec, A., Dijkshoorn, L. & Brisse, S. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 5, e10034 (2010).
CLSI Performance standards for antimicrobial susceptibility testing. Twenty-third Informational Supplement M100-S23 Committee for Clinical Laboratory Standards Institute, CLSI, Wayne, PA, USA, (2013).
Brown, S. & Amyes, S. OXA (beta)-lactamases in Acinetobacter: the story so far. J. Antimicrob. Chemother. 57, 1–3 (2006).
Peleg, A. Y., Seifert, H. & Paterson, D. L. Acinetobacter baumannii: emergence of a successfulpathogen. Clin. Microbiol. Rev. 21, 538–582 (2008).
Srinivasan, V. B., Rajamohan, G., Pancholi, P., Stevenson, K., Tadesse, D. & Patchanee, P. Genetic relatedness and molecular characterization of multidrug resistant Acinetobacter baumannii isolated in central Ohio, USA. Ann. Clin. Microbiol. Antimicrob. 8, 21 (2009).
Zarrilli, R. et al. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumanniiisolates from a Lebanese Hospital. Antimicrob. Agents Chemother. 52, 4115–4120 (2008).
Kempf, M. & Rolain, J. M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents 39, 105–114 (2012).
Adams-Haduch, J. M. et al. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob. Agents Chemother. 52, 3837–3843 (2008).
Pendleton, J. N., Gorman, S. P. & Gilmore, B. F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 11, 297–308 (2013).
de Oliveira, A. C. & Damasceno, Q. S. Surfaces of the hospital environment as possible deposits of resistant bacteria: a review. Rev. Esc. Enferm. USP. 44, 1118–1123 (2010).
Fu, Y., Zhou, J & Zhou, H. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J. Antimicrob. Chemother. 65, 644–650 (2010).
Durmaz, R., Otlu, B. & Koksal, F. The optimization of a rapid pulsed-field gel electrophoresis protocol for the typing of Acinetobacter baumannii Escherichia coli and Klebsiella spp. Jpn. J. Infect. Dis. 62, 372–377 (2009).
Hamouda, A., Evans, B. A., Towner, K. J. & Amyes, S. G. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of bla(OXA-51-like) genes. J. Clin. Microbiol. 48, 2476–2483 (2010).
Tenover, F. C., Arbeit, R. D. & Goering, R. V. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33, 2233–2239 (1995).
Karah, N., Sundsfjord, A., Towner, K. & Samuelsen, O. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist. Updat. 15, 237–247 (2012).
Lee, C. M., Liao, C. H. & Lee, W. S. Outbreak of Klebsiella pneumoniae carbapenemase-2-producing K. pneumoniae sequence type 11 in Taiwan in 2011. Antimicrob. Agents Chemother. 56, 5016–5022 (2012).
Munoz-Price, L. S. & Weinstein, R. A. Acinetobacter infection. N. Engl. J. Med. 358, 1271–1281 (2008).
Jean, S. S. & Hsueh, P. R. High burden of antimicrobial resistance in Asia. Int. J. Antimicrob. Agents 37, 291–295 (2011).
Villegas, M. V. & Hartstein, A. I. Acinetobacter outbreaks, 1977–2000. Infect. Control Hosp. Epidemiol. 24, 284–295 (2003).
Poirel, L. & Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12, 826–836 (2006).
Koh, T. H., Sng, L. H., Wang, G. C. Y., Hsu, L. Y. & Zhao, Y. IMP-4 and OXA beta-lactamases in Acinetobacter baumannii from Singapore. J. Antimicrob. Chemother. 59, 627–632 (2007).
Mendes, R. E., Bell, J. M., Turnidge, J. D., Castanheira, M. & Jones, R. N. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia- Pacific nations: report from the SENTRY Surveillance Program. J. Antimicrob. Chemother. 63, 55–59 (2009).
M.-H., Lee., T.-L., Chen & Lee, Y.-T. Dissemination of multidrug-resistant Acinetobacter baumannii carrying BlaOxA-23 from hospitals in central Taiwan. J. Microbiol. Immunol. Infect. 46, 419–424 (2013).
Wang, X., Zong, Z. & Lu, X. Tn2008 is a major vehicle carrying bla(OXA-23) in Acinetobacter baumannii from China. Diagn. Microbiol. Infect. Dis. 69, 218–222 (2011).
Mezzatesta, M. L., D'Andrea, M. M. & Migliavacca, R. Epidemiological characterization and distribution of carbapenem-resistant Acinetobacter baumannii clinical isolates in Italy. Clin. Microbiol. Infect. 18, 160–166 (2012).
Morgan, D. J., Liang, S. Y. & Smith, C. L. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect. Control Hosp. Epidemiol. 31, 716–721 (2010).
Durante-Mangoni, E. & Zarrilli, R. Global spread of drug-resistant Acinetobacter baumannii: molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 6, 407–422 (2011).
Singh, A., Goering, R. V., Simjee, S., Foley, S. L. & Zervos, M. J. Application of molecular techniques to the study of hospital infection. Clin. Microbiol. Rev. 19, 512–530 (2006).
Tan, R., Liu, J. & Li, M. Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university-affiliated hospital in Shanghai. J. Microbiol. Immunol. Infect. 47, 87–94 (2014).
Thom, K. A., Johnson, J. K., Lee, M. S. & Harris, A. D. Environmental contamination because of multidrug-resistant Acinetobacter baumannii surrounding colonized or infected patients. Am. J. Infect. Control 39, 711–715 (2011).
Dijkshoorn, L., Nemec, A. & Seifert, H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5, 939–951 (2007).
Wang, X., Qiao, F., Yu, R., Gao, Y. & Zong, Z. Clonal diversity of Acinetobacter baumannii clinical isolates revealed by a snapshot study. BMC Microbiol. 13, 234 (2013).
Acknowledgements
This study was supported by National Natural Science Foundation of China. (Grant 81371874). We thank Professor Hongsheng Zhu for a critical reading and revision of the manuscript and Demei Zhu and Fupin Hu for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ying, C., Li, Y., Wang, Y. et al. Investigation of the molecular epidemiology of Acinetobacter baumannii isolated from patients and environmental contamination. J Antibiot 68, 562–567 (2015). https://doi.org/10.1038/ja.2015.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2015.30
This article is cited by
-
Characterization of class 1 integrons in metallo-β-lactamase-producing Acinetobacter baumannii isolates from hospital environment
BMC Research Notes (2023)
-
Molecular typing of multi-drug resistant Acinetobacter baumannii isolates from clinical and environmental specimens in three Iranian hospitals by pulsed field gel electrophoresis
BMC Microbiology (2020)
-
Antibiotic-resistant clones in Gram-negative pathogens: presence of global clones in Korea
Journal of Microbiology (2019)
-
Genotyping and distribution of putative virulence factors and antibiotic resistance genes of Acinetobacter baumannii strains isolated from raw meat
Antimicrobial Resistance & Infection Control (2018)
-
Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii
European Journal of Clinical Microbiology & Infectious Diseases (2018)