Abstract
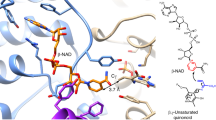
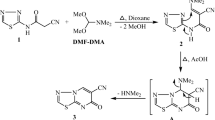
The piericidin family of microbial metabolites features a 4-pyridinol core linked with a methylated polyketide side chain. Piericidins are exclusively produced by actinomycetes, especially members of the genus Streptomyces. The close structural similarity with coenzyme Q renders the piericidins important NADH-ubiquinone oxidoreductase (complex I) inhibitors in the mitochondrial electron transport chain. Because of the significant activities of the piericidins, which include insecticidal, antimicrobial and antitumor effects, total syntheses of the piericidins were developed using various synthetic strategies. The biosynthetic origin of this class has also been the subject of investigation. This review covers the isolation and structure determination of the natural piericidins, their chemical modification, the total syntheses of natural and unnatural analogs, their biosynthesis, and reported biological activities together with structure–activity relationships. Given the fundamental biology of this class of metabolites, the piericidin family will likely continue to attract attention as biological probes of important biosynthetic processes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hall, C. et al. Piericidin A: a new inhibitor of mitochondrial electron transport. Biochem. Biophys. Res. Commun. 25, 373–377 (1966).
Yoshida, S. & Takahashi, N. Piericidins–naturally occurring inhibitors against mitochondrial respiration. Heterocycles 10, 425–467 (1978).
Tamura, S. et al. Isolation and physiological activities of piericidin a, a natural insecticide produced by Streptomyces. Agr. Biol. Chem. 27, 576–582 (1963).
Takahashi, N., Suzuki, A. & Tamura, S. Chemical structure of piericidin A. 2. Ozonolysis products of piericidin A derivatives. Agr. Biol. Chem. 27, 798–805 (1963).
Takahashi, N., Suzuki, A., Miyamoto, S., Tamura, S. & Mori, R. Chemical structure of piericidin A. 1. Functional groups. Agr. Biol. Chem. 27, 583–589 (1963).
Takahashi, N., Suzuki, A. & Tamura, S. Structure of piericidin A. J. Am. Chem. Soc. 87, 2066–2068 (1965).
Takahashi, N., Suzuki, A. & Tamura, S. Chemical structure of piericidin A. 3. Structures of piericidin A and octahydropiericidin A. Agr. Biol. Chem. 30, 1–12 (1966).
Suzuki, A., Takahashi, N. & Tamura, S. Chemical structure of piericidin A. 4. Structural confirmation for pyridine ring in piericidin a through synthesis. Agr. Biol. Chem. 30, 13–17 (1966).
Takahashi, N., Suzuki, A., Kimura, Y., Miyamoto, S. & Tamura, S. Structure of piericidin B and stereochemistry of piericidins. Tetrahedron Lett. 8, 1961–1964 (1967).
Takahashi, N., Yoshida, S., Suzuki, A. & Tamura, S. Chemical structure of piericidin A. 6. Stereochemistry. Agr. Biol. Chem. 32, 1108–1114 (1968).
Yoshida, S., Shiraishi, S. & Takahashi, N. Structural revision of piericidin A by combination of CMR spectroscopic and biosynthetic studies. Agr. Biol. Chem. 41, 587–591 (1977).
Yoshida, S., Shiraishi, S., Fujita, K. & Takahashi, N. Biosynthetic studies on piericidin A and its structural revision. Tetrahedron Lett. 16, 1863–1866 (1975).
Jansen, R. & Hofle, G. Revised stereochemistry of piericidin A1. Tetrahedron Lett. 24, 5485–5486 (1983).
Cox, C. M. & Whiting, D. A. Stereoselective synthesis of a synthon for the natural electron–transfer inhibitors myxalamide D and piericidin A. J. Chem. Soc. Perkin Trans. 1, 660–662 (1991).
Cox, C. M. & Whiting, D. A. Synthetic studies on electron–transport inhibitors. 1. Chiral synthesis of a synthon for myxalamide D, piericidin A, and the actinopyrones. J. Chem. Soc. Perkin Trans. 1, 1901–1905 (1991).
Yoshida, S., Yoneyama, K., Shiraishi, S., Watanabe, A. & Takahashi, N. Chemical structures of new piericidins produced by Streptomyces pactum. Agr. Biol. Chem. 41, 855–862 (1977).
Yoshida, S., Yoneyama, K., Shiraishi, S., Watanabe, A. & Takahashi, N. Isolation and physical–properties of new piericidins produced by Streptomyces pactum. Agr. Biol. Chem. 41, 849–853 (1977).
Nishioka, H. et al. Isolation and structure determination of a novel phosphatidylinositol turnover inhibitor, piericidin B1 N-oxide. J. Antibiot. 44, 1283–1285 (1991).
Nishioka, H. et al. Isolation and structure determination of novel phosphatidylinositol turnover inhibitors, piericidin B5 and B5 N-oxide, from Streptomyces sp. J. Antibiot. 46, 564–568 (1993).
Urakawa, A. et al. IT-143-A and B, novel piericidin group antibiotics produced by Streptomyces sp. J. Antibiot. 49, 1052–1055 (1996).
Ueda, J. Y. et al. A novel nuclear export inhibitor JBIR-02, a new piericidin discovered from Streptomyces sp. ML55. J. Antibiot. 60, 459–462 (2007).
Hoecker, J. & Gademann, K. Enantioselective total syntheses and absolute configuration of JBIR-02 and Mer-A2026B. Org. Lett. 15, 670–673 (2013).
Kubota, N. K. et al. Piericidins C5 and C6: new 4-pyridinol compounds produced by Streptomyces sp. and Nocardioides sp. Bioorg. Med. Chem. 11, 4569–4575 (2003).
Hayakawa, Y. et al. Piericidins C7 and C8, new cytotoxic antibiotics produced by a marine Streptomyces sp. J. Antibiot. 60, 196–200 (2007).
Hayakawa, Y. et al. Structures of new cytotoxic antibiotics, piericidins C7 and C8. J. Antibiot. 60, 201–203 (2007).
Kimura, K., Takahashi, H., Miyata, N., Yoshihama, M. & Uramoto, M. New piericidin antibiotics, 7-demethylpiericidin A1 and 7-demethyl-3'-rhamnopiericidin A1. J. Antibiot. 49, 697–699 (1996).
Chen, Y. et al. Elucidating hydroxylation and methylation steps tailoring piericidin A1 biosynthesis. Org. Lett. 16, 736–739 (2014).
Matsumoto, M. et al. New piericidin glucosides, glucopiericidins A and B. J. Antibiot. 40, 149–156 (1987).
Funayama, S. et al. Novel cytocidal antibiotics, glucopiericidinols A1 and A2. Taxonomy, fermentation, isolation, structure elucidation and biological characteristics. J. Antibiot. 42, 1734–1740 (1989).
Mori, H., Funayama, S., Sudo, Y., Komiyama, K. & Omura, S. A new antibiotic, 13-hydroxyglucopiericidin A. Isolation, structure elucidation and biological characteristics. J. Antibiot. 43, 1329–1331 (1990).
Kimura, K. et al. A new piericidin rhamnoside, 3'-rhamnopiericidin A1. J. Antibiot. 43, 1341–1343 (1990).
Iwasaki, H., Kamisango, K., Kuboniwa, H., Sasaki, H. & Matsubara, S. 3'-Deoxytalopiericidin-A1, a novel analog of antitumor antibiotics from oligotroph. J. Antibiot. 44, 451–452 (1991).
Shaaban, K. A., Helmke, E., Kelter, G., Fiebig, H. H. & Laatsch, H. Glucopiericidin C: a cytotoxic piericidin glucoside antibiotic produced by a marine Streptomyces isolate. J. Antibiot. 64, 205–209 (2011).
Schmidtchen, F. P. & Rapoport, H. Polyprenylpyridinols. Synthesis of piericidin analogues. J. Am. Chem. Soc. 99, 7014–7019 (1977).
Yoshida, S., Nagao, Y. & Takahashi, N. Synthesis of piericidin analogs, inhibitors on electron–transport system in mitochondria. Agr. Biol. Chem. 44, 2913–2920 (1980).
Yoshida, S., Nagao, Y., Watanabe, A. & Takahashi, N. Structure–activity relationship in piericidins inhibitors on the electron–transport system in mitochondria. Agr. Biol. Chem. 44, 2921–2924 (1980).
Kominato, K. et al. Mer-A2026A and B, novel piericidins with vasodilating effect. II. Physico–chemical properties and chemical structures. J. Antibiot. 48, 103–105 (1995).
Ono, M., Yoshida, N. & Akita, H. Synthetic study of piericidins. 2. Synthesis of piericidin analogues. Chem. Pharm. Bull. 45, 1745–1750 (1997).
Schnermann, M. J. et al. Total synthesis of piericidin A1 and B1 and key analogues. J. Am. Chem. Soc. 128, 11799–11807 (2006).
Schnermann, M. J. & Boger, D. L. Total synthesis of piericidin A1 and B1. J. Am. Chem. Soc. 127, 15704–15705 (2005).
Keaton, K. A. & Phillips, A. J. Titanium(II)–mediated cyclizations of (silyloxy)enynes: A total synthesis of (–)-7-demethylpiericidin A1. J. Am. Chem. Soc. 128, 408–409 (2006).
Kikuchi, R., Fujii, M. & Akita, H. Total synthesis of (+)-piericidin A1 and (–)-piericidin B1. Tetrahedron Asymmetry 20, 1975–1983 (2009).
Kikuchi, R., Fujii, M. & Akita, H. Synthetic study of piericidin A1 and B1. J. Mol. Catal. B Enzym. 62, 125 (2010).
Lipshutz, B. H. & Amorelli, B. Total synthesis of piericidin A1. Application of a modified Negishi carboalumination-nickel-catalyzed cross-coupling. J. Am. Chem. Soc. 131, 1396–1397 (2009).
Ono, M., Yoshida, N., Kokubu, Y., Sato, E. & Akita, H. Synthetic study of piericidins.1. Synthesis of the side chain of piericidin B1. Chem. Pharm. Bull. 45, 1428–1434 (1997).
Kimura, Y., Takahashi, N. & Tamura, S. Biosynthesis of piericidins A and B by Streptomyces mobaraensis. Agr. Biol. Chem. 33, 1507 (1969).
Tanabe, M. & Seto, H. Biosynthetic studies with carbon 13. Piericidin A. J. Org. Chem. 35, 2087–2088 (1970).
Liu, Q. et al. Elucidation of piericidin A1 biosynthetic locus revealed a thioesterase–dependent mechanism of alpha–pyridone ring formation. Chem. Biol. 19, 243–253 (2012).
Degli Esposti, M. et al. Complex I and complex III of mitochondria have common inhibitors acting as ubiquinone antagonists. Biochem. Biophys. Res. Commun. 190, 1090–1096 (1993).
Gutman, M., Beinert, H. & Singer, T. P. Studies on respiratory chain-linked reduced nicotinamide-adenine dinucleotide dehydrogenase. 20. Relation of respiratory chain-linked reduced nicotinamide-adenine dinucleotide dehydrogenase to energy-coupling site-1. Biochemistry 11, 556–562 (1972).
Friedrich, T. et al. Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone–binding site of bacterial glucose:ubiquinone oxidoreductase. Eur. J. Biochem. 219, 691–698 (1994).
Lambert, A. J. & Brand, M. D. Inhibitors of the quinone–binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279, 39414–39420 (2004).
Prieur, I., Lunardi, J. & Dupuis, A. Evidence for a quinone binding site close to the interface between NUOD and NUOB subunits of Complex I. Biochim. Biophys. Acta 1504, 173–178 (2001).
Gutman, M. & Kliatchko, S. Mechanism of inhibition by ubicidin – inhibitor with piericidin ring structure and ubiquinone side chain. FEBS Lett. 67, 348–353 (1976).
Degli Esposti, M., Ghelli, A., Ratta, M., Cortes, D. & Estornell, E. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem. J. 301, 161–167 (1994).
Mitsui, T., Fukami, J. I., Fukunaga, K., Takahashi, N. & Tamura, S. Studies on piericidin – Antagonistic effect of vitamin-K3 on respiratory chain of insects and mammals in presence of piericidin A. Agr. Biol. Chem. 34, 1101–1109 (1970).
Chung, K., Cho, K., Asami, Y., Takahashi, N. & Yoshida, S. in Bioenergetics (eds Kim, C. & Ozawa, T.) 65–81 (Springer, 1990).
Miyoshi, H. Structure-activity relationships of some complex I inhibitors. Biochim. Biophys. Acta Bioenerg. 1364, 236–244 (1998).
Degli Esposti, M. Inhibitors of NADH–ubiquinone reductase: an overview. Biochim. Biophys. Acta 1364, 222–235 (1998).
Chung, K. H., Cho, K. Y., Asami, Y., Takahashi, N. & Yoshida, S. New 4-hydroxypyridine and 4-hydroxyquinoline derivatives as inhibitors of NADH-ubiquinone reductase in the respiratory-chain. Z. Naturforsch. C. 44, 609–616 (1989).
Horgan, D. J., Ohno, H., Singer, T. P. & Casida, J. E. Studies on respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 15. Interactions of piericidin with mitochondrial respiratory chain. J. Biol. Chem. 243, 5967–5976 (1968).
Takahashi, N. et al. Isolation structure and physiological activities of piericidin B nature insecticide produced by a Streptomyces. Agr. Biol. Chem. 32, 1115–1122 (1968).
Lummen, P. Complex I inhibitors as insecticides and acaricides. Biochim. Biophys. Acta 1364, 287–296 (1998).
Fato, R. et al. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim. Biophys. Acta 1787, 384–392 2009.
Ohnishi, S. T. et al. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J. Bioenerg. Biomembr. 37, 1–15 (2005).
Lee, K. K. et al. Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes. Free Radic. Biol. Med. 65, 584–594 (2013).
Ikezawa, N., Ifuku, K., Endo, T. & Sato, F. Inhibition of photosystem II of spinach by the respiration inhibitors piericidin A and thenoyltrifluoroacetone. Biosci. Biotechnol. Biochem. 66, 1925–1929 (2002).
Hollerhage, M. et al. Piericidin A aggravates Tau pathology in P301S transgenic mice. PLoS ONE 9, e113557 (2014).
Choi, W. S., Palmiter, R. D. & Xia, Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. J. Cell Biol. 192, 873–882 (2011).
Jeso, V., Yang, C., Cameron, M. D., Cleveland, J. L. & Micalizio, G. C. Synthesis and SAR of Lehualide B: a marine–derived natural product with potent anti–multiple myeloma activity. ACS Chem. Biol. 8, 1241–1252 (2013).
Takatsuki, A., Tamura, G. & Arima, K. Antiviral and antitumor antibiotics. XIV. Effects of ascochlorin and other respiration inhibitors on multiplication of Newcastle disease virus in cultured cells. Appl. Microbiol. 17, 825–829 (1969).
Kominato, K. et al. Mer-A2026A and B, novel piericidins with vasodilating effect. I. Producing organism, fermentation, isolation and biological properties. J. Antibiot. 48, 99–102 (1995).
Kroiss, J. et al. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6, 261–263 (2010).
Sawyer, S. T. & Cohen, S. Enhancement of calcium-uptake and phosphatidylinositol turnover by epidermal growth factor in A431 cells. Biochemistry 20, 6280–6286 (1981).
Habenicht, A. J. R. et al. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet–derived growth factor. J. Biol. Chem. 256, 2329–2335 (1981).
Nishioka, H. et al. Isolation and structure determination of a novel phosphatidylinositol turnover inhibitor, piericidin B1 N-Oxide. J. Antibiot. 44, 1283–1285 (1991).
Nishioka, H. et al. Antitumor effect of piericidin B1 N-oxide. J. Antibiot. 47, 447–452 (1994).
Ahn, S. C. et al. Inhibition of PDGF–induced phosphoinositide–turnover by glucopiericidin A. Biochem. Mol. Biol. Int. 37, 125–132 (1995).
Debbas, M. & White, E. Wild-type P53 mediates apoptosis by E1a, which is inhibited by E1b. Gene Dev. 7, 546–554 (1993).
Kitagawa, M., Ikeda, S., Tashiro, E., Soga, T. & Imoto, M. Metabolomic identification of the target of the filopodia protrusion inhibitor glucopiericidin A. Chem. Biol. 17, 989–998 (2010).
Hwang, J. H. et al. Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down–regulator. J. Cell Physiol. 215, 243–250 (2008).
Kuo, J. F., Dill, I. K. & Holmlund, C. E. Effects of piericidin a on metabolism of isolated adipose cells. Biochem. Pharmacol. 17, 867–872 (1968).
Magae, J. et al. Screening for specific inhibitors of phagocytosis of thioglycollate-elicited macrophages. Biosci. Biotechnol. Biochem. 58, 104–107 (1994).
Ahn, S. C. et al. Screening of interleukin–2 production inhibitor with mouse thymoma EL4 cells. J. Antibiot. 55, 1013–1015 (2002).
Duncan, M. C. et al. An NF-κB-based high-throughput screen identifies piericidins as inhibitors of the Yersinia pseudotuberculosis type III secretion system. Antimicrob. Agents Chemother. 58, 1118–1126 (2014).
Acknowledgements
This work is a result of financial support from the National Institutes of Health, National Cancer Institute under grant CA044848 (to WF) and from the China Scholarship Council (to XZ). This article is dedicated to the fond memory of the late professor Lester Mitscher, a great scholar, teacher and emeritus editor of this Journal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, X., Fenical, W. The unique chemistry and biology of the piericidins. J Antibiot 69, 582–593 (2016). https://doi.org/10.1038/ja.2016.71
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2016.71
This article is cited by
-
Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
Nature Chemistry (2022)
-
The immunotoxicity of ten insecticides against insect hemocyte cells in vitro
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Special issue dedicated to William Fenical: a pioneer in marine/marine-derived microbial chemistry
The Journal of Antibiotics (2020)
-
Glycosylated piericidins from an endophytic streptomyces with cytotoxicity and antimicrobial activity
The Journal of Antibiotics (2018)