Abstract
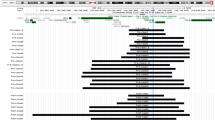
Partial duplications involving the long arm of the X chromosome are associated with mental retardation, short stature, microcephaly, hypopituitarism and a wide range of physical findings. We identified an inherited Xq26.2-Xq26.3 duplication in two brothers with severe mental retardation, hypotonia, growth delay, craniofacial disproportion and dental malocclusion. Chromosome analysis was normal and multiplex ligation-dependent probe amplification analysis detected duplication on Xq26. Further characterization by array comparative genomic hybridization and quantitative PCR helped to determine proximal and distal duplication breakpoints giving a size of approximately 2.8 Mb. The duplication encompasses 24 known genes, including the X-linked mental retardation genes ARHGEF6, PHF6, HPRT1 and SLC9A6. Clinical and molecular characterization of Xq duplications will shed more light into the phenotypic implication of functional disomy of X-chromosome genes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shaw-Smith, C., Redon, R., Rickman, L., Rio, M., Willatt, L., Fiegler, H. et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J. Med. Genet. 41, 241–248 (2004).
Madrigal, I., Rodriguez-Revenga, L., Armengol, L., Gonzalez, E., Rodriguez, B., Badenas, C. et al. X-chromosome tiling path array detection of copy number variants in patients with chromosome X-linked mental retardation. BMC Genomics 8, 443 (2007).
Madrigal, I., Rodriguez-Revenga, L., Badenas, C., Sanchez, A., Martinez, F., Fernandez, I. et al. MLPA as first screening method for the detection of microduplications and microdeletions in patients with X-linked mental retardation. Genet. Med. 9, 117–122 (2007).
Froyen, G., Van Esch, H., Bauters, M., Hollanders, K., Frints, S. G., Vermeesch, J. R. et al. Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: important role for increased gene dosage of XLMR genes. Hum. Mutat. 28, 1034–1042 (2007).
Lugtenberg, D., de Brouwer, A. P., Kleefstra, T., Oudakker, A. R., Frints, S. G., Schrander-Stumpel, C. T. et al. Chromosomal copy number changes in patients with non-syndromic X linked mental retardation detected by array CGH. J. Med. Genet. 43, 362–370 (2006).
Steinbach, P., Horstmann, W. & Scholz, W. Tandem duplication dup(X)(q13q22) in a male proband inherited from the mother showing mosaicism of X-inactivation. Hum. Genet. 54, 309–313 (1980).
Thode, A., Partington, M. W., Yip, M. Y., Chapman, C., Richardson, V. F. & Turner, G. A new syndrome with mental retardation, short stature and an Xq duplication. Am. J. Med. Genet. 30, 239–250 (1988).
Schmidt, M., Du Sart, D., Kalitsis, P., Leversha, M., Dale, S., Sheffield, L. et al. Duplications of the X chromosome in males: evidence that most parts of the X chromosome can be active in two copies. Hum. Genet. 86, 519–521 (1991).
Muscatelli, F., Verna, J. M., Philip, N., Moncla, A., Mattei, M. G., Mattei, J. F. et al. Physical mapping of an Xq-proximal interstitial duplication in a male. Hum. Genet. 88, 691–694 (1992).
Vasquez, A. I., Rivera, H., Bobadilla, L. & Crolla, J. A. A familial Xp+ chromosome, dup (Xq26.3—>qter). J. Med. Genet. 32, 891–893 (1995).
Novelli, A., Bernardini, L., Salpietro, D. C., Briuglia, S., Merlino, M. V., Mingarelli, R. et al. Disomy of distal Xq in males: case report and overview. Am. J. Med. Genet. A 128A, 165–169 (2004).
Lagerstrom-Fermer, M., Sundvall, M., Johnsen, E., Warne, G. L., Forrest, S. M., Zajac, J. D. et al. X-linked recessive panhypopituitarism associated with a regional duplication in Xq25-q26. Am. J. Hum. Genet. 60, 910–916 (1997).
Hol, F. A., Schepens, M. T., van Beersum, S. E., Redolfi, E., Affer, M., Vezzoni, P. et al. Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics 69, 174–181 (2000).
Lachlan, K. L., Collinson, M. N., Sandford, R. O., van Zyl, B., Jacobs, P. A. & Thomas, N. S. Functional disomy resulting from duplications of distal Xq in four unrelated patients. Hum. Genet. 115, 399–408 (2004).
Akiyama, M., Kawame, H., Ohashi, H., Tohma, T., Ohta, H., Shishikura, A. et al. Functional disomy for Xq26.3-qter in a boy with an unbalanced t(X;21)(q26.3;p11.2) translocation. Am. J. Med. Genet. 99, 111–114 (2001).
Solomon, N. M., Ross, S. A., Forrest, S. M., Thomas, P. Q., Morgan, T., Belsky, J. L. et al. Array comparative genomic hybridisation analysis of boys with X-linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J. Med. Genet. 44, e75 (2007).
Madrigal, I., Carrio, A., Gomez, C., Rozman, M., Esteve, J., Nomdedeu, B. et al. Fluorescence in situ hybridization studies using BAC clones of the EVI1 locus in hematological malignancies with 3q rearrangements. Cancer Genet. Cytogenet. 170, 115–120 (2006).
Rodriguez-Revenga, L., Madrigal, I., Badenas, C., Xuncla, M., Jimenez, L. & Mila, M. Premature ovarian failure and fragile X female premutation carriers: no evidence for a skewed X-chromosome inactivation pattern. Menopause 16, 944–949 (2009).
Carrel, L. & Willard, H. F. An assay for X inactivation based on differential methylation at the fragile X locus, FMR1. Am. J. Med. Genet. 64, 27–30 (1996).
Zhang, A., Weaver, D. D. & Palmer, C. G. Molecular cytogenetic identification of four X chromosome duplications. Am. J. Med. Genet. 68, 29–38 (1997).
Cheng, S. F., Rauen, K. A., Pinkel, D., Albertson, D. G. & Cotter, P. D. Xq chromosome duplication in males: clinical, cytogenetic and array CGH characterization of a new case and review. Am. J. Med. Genet. A 135, 308–313 (2005).
Lugtenberg, D., Kleefstra, T., Oudakker, A. R., Nillesen, W. M., Yntema, H. G., Tzschach, A. et al. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur. J. Hum. Genet. 17, 444–453 (2009).
Wolf, N. I., Sistermans, E. A., Cundall, M., Hobson, G. M., Davis-Williams, A. P., Palmer, R. et al. Three or more copies of the proteolipid protein gene PLP1 cause severe Pelizaeus–Merzbacher disease. Brain 128, 743–751 (2005).
Kutsche, K., Yntema, H., Brandt, A., Jantke, I., Nothwang, H. G., Orth, U. et al. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat. Genet. 26, 247–250 (2000).
Carter, M. T., Picketts, D. J., Hunter, A. G. & Graham, G. E. Further clinical delineation of the Borjeson–Forssman–Lehmann syndrome in patients with PHF6 mutations. Am. J. Med. Genet. A 149A, 246–250 (2009).
Tvrdik, T., Marcus, S., Hou, S. M., Falt, S., Noori, P., Podlutskaja, N. et al. Molecular characterization of two deletion events involving Alu-sequences, one novel base substitution and two tentative hotspot mutations in the hypoxanthine phosphoribosyltransferase (HPRT) gene in five patients with Lesch–Nyhan syndrome. Hum. Genet. 103, 311–318 (1998).
Gilfillan, G. D., Selmer, K. K., Roxrud, I., Smith, R., Kyllerman, M., Eiklid, K. et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am. J. Hum. Genet. 82, 1003–1010 (2008).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 (1990).
Node-Langlois, R., Muller, D. & Boda, B. Sequential implication of the mental retardation proteins ARHGEF6 and PAK3 in spine morphogenesis. J. Cell Sci. 119, 4986–4993 (2006).
Lower, K. M., Turner, G., Kerr, B. A., Mathews, K. D., Shaw, M. A., Gedeon, A. K. et al. Mutations in PHF6 are associated with Borjeson–Forssman–Lehmann syndrome. Nat. Genet. 32, 661–665 (2002).
Borjeson, M., Forssman, H. & Lehmann, O. An X-linked, recessively inherited syndrome characterized by grave mental deficiency, epilepsy, and endocrine disorder. Acta. Med. Scand. 171, 13–21 (1962).
Keebaugh, A. C., Sullivan, R. T. & Thomas, J. W. Gene duplication and inactivation in the HPRT gene family. Genomics 89, 134–142 (2007).
Wakabayashi, S., Shigekawa, M. & Pouyssegur, J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol. Rev. 77, 51–74 (1997).
Counillon, L. & Pouyssegur, J. The expanding family of eucaryotic Na(+)/H(+) exchangers. J. Biol. Chem. 275, 1–4 (2000).
Laumonnier, F., Ronce, N., Hamel, B. C., Thomas, P., Lespinasse, J., Raynaud, M. et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am. J. Hum. Genet. 71, 1450–1455 (2002).
Woods, K. S., Cundall, M., Turton, J., Rizotti, K., Mehta, A., Palmer, R. et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am. J. Hum. Genet. 76, 833–849 (2005).
Gecz, J., Baker, E., Donnelly, A., Ming, J. E., McDonald-McGinn, D. M., Spinner, N. B. et al. Fibroblast growth factor homologous factor 2 (FHF2): gene structure, expression and mapping to the Borjeson–Forssman–Lehmann syndrome region in Xq26 delineated by a duplication breakpoint in a BFLS-like patient. Hum. Genet. 104, 56–63 (1999).
Plenge, R. M., Stevenson, R. A., Lubs, H. A., Schwartz, C. E. & Willard, H. F. Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am. J. Hum. Genet. 71, 168–173 (2002).
Mohandas, T., Geller, R. L., Yen, P. H., Rosendorff, J., Bernstein, R., Yoshida, A. et al. Cytogenetic and molecular studies on a recombinant human X chromosome: implications for the spreading of X chromosome inactivation. Proc. Natl Acad. Sci. USA 84, 4954–4958 (1987).
Acknowledgements
We thank Fundación Ramón Areces (V-2006-FRARECES) and all members of this family for their cooperation. The ‘CIBER de Enfermedades Raras’ is an initiative of the ISCIII. We thank Dr Beatriz Gómez for revising the clinical section of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madrigal, I., Fernández-Burriel, M., Rodriguez-Revenga, L. et al. Xq26.2-q26.3 microduplication in two brothers with intellectual disabilities: clinical and molecular characterization. J Hum Genet 55, 822–826 (2010). https://doi.org/10.1038/jhg.2010.119
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2010.119
Keywords
This article is cited by
-
Genomic imbalances defining novel intellectual disability associated loci
Orphanet Journal of Rare Diseases (2019)
-
A Christianson syndrome-linked deletion mutation (∆287ES288) in SLC9A6 disrupts recycling endosomal function and elicits neurodegeneration and cell death
Molecular Neurodegeneration (2016)
-
X-linked congenital ptosis and associated intellectual disability, short stature, microcephaly, cleft palate, digital and genital abnormalities define novel Xq25q26 duplication syndrome
Human Genetics (2014)