Abstract
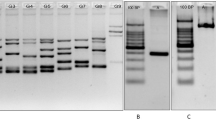
Mutations in BRCA genes elevate risk for breast and ovarian cancer. These mutations are population specific. As there are no data on BRCA mutation screening on larger number of probands in Serbia to date, aim of this study was to determine types and frequencies of BRCA mutations in individuals from high-risk families from Serbia, as well as to determine which BRCA mutations may be considered as founder for Serbian population. We analyzed 94 probands and detected 9 frameshift mutations in 12 individuals, 1 benign BRCA2 nonsense mutation and numerous missense and synonymous mutations in both genes. Frequency of frameshift mutations is 12.77%. In addition to two novel mutations detected in our population we reported previously, we detected another novel mutation—c.7283delT in BRCA2 exon 14. None of the detected deleterious mutations may be considered as founder mutations for Serbian population, as each of them was found in no more than two high-risk families. This mutation diversity is most probably due to high migration rate in history of this part of Europe. Interpretation of genetic testing results with missense mutations of unknown clinical importance is very challenging and should be approached with caution, using all available data sources for results’ interpretation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hall, J. M., Lee, M. K., Newman, B., Morrow, J. E., Anderson, L. A., Huey, B. et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250, 1684–1689 (1990).
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Scully, R., Ganesan, S., Brown, M., De Caprio, J. A., Cannistra, S. A., Feunteun, J. et al. Location of BRCA1 in human breast and ovarian cell lines. Science 272, 123–125 (1996).
Meza, J. E., Brzovic, P. S., King, M. C. & Klevit, R. E. Mapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1. J. Biol. Chem. 274, 5659–5665 (1999).
Chen, C. F., Li, S., Chen, Y., Chen, P. L., Sharp, Z. D. & Lee, W. H. The nuclear localization sequences of the BRCA1 protein interact with the importin-α subunit of the nuclear transport signal receptor. J. Biol. Chem. 271, 32863–32868 (1996).
Paull, T. T., Cortez, D., Bowers, B., Elledge, S. J. & Gellert, M. From the cover: direct DNA binding by Brca1. Proc. Natl Acad. Sci. USA 98, 6086–6091 (2001).
Koonin, E. V., Altschul, S. F. & Bork, P. BRCA1 protein products… Functional motifs…. Nat. Genet. 13, 266–268 (1996).
Thorslund, T. & West, S. C. BRCA2: a universal recombinase regulator. Oncogene 26, 7720–7730 (2007).
Teng, L. S., Zheng, Y. & Wang, H. H. BRCA1/2 associated hereditary breast cancer. J. Zhejiang. Univ. Sci. B 9, 85–89 (2008).
Chen, C. F., Chen, P. L., Zhong, Q., Sharp, Z. D. & Lee, W. H. Expression of BRC repeats in breast cancer cells disrupts the BRCA2eRad51 complex and leads to radiation hypersensitivity and loss of G2/M checkpoint control. J. Biol. Chem. 274, 32931–32935 (1999).
Yang, H., Jeffrey, P. D., Miller, J., Kinnucan, E., Sun, Y., Thoma, N. H. et al. BRCA2 function in DNA binding and recombination from a BRCA2–DSS1–ssDNA structure. Science 297, 1837–1848 (2002).
Spain, B. H., Larson, C. J., Shihabuddin, L.S., Gage, F. H. & Verma, I. M. Truncated BRCA2 is cytoplasmic: implications for cancer-linked mutations. Proc. Natl Acad. Sci. USA 96, 13920–13925 (1999).
Huen, M. S. Y., Sy, S. M. H. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell. Biol. 11, 138–148 (2010).
Gudmundsdottir, K. & Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25, 5864–5874 (2006).
Lindor, N. M., McMaster, M. L., Lindor, C. J. & Greene, M. H. National Cancer Institute, Division of Cancer Prevention, Community Oncology and Prevention Trials Research Group. Concise handbook of familial cancer susceptibility syndromes—second edition. J. Natl Cancer Inst. Monogr. 38, 1–93 (2008).
Ferla, R., Calo, V., Cascio, S., Rinaldi, G., Badalamenti, G., Carreca, I. et al. Founder mutations in BRCA1 and BRCA2 genes. Ann. Oncol. 18 (Suppl 6), vi93–vi98 (2007).
Abeliovich, D., Kaduri, L., Lerer, I., Weinberg, N., Amir, G., Sagi, M. et al. The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am. J. Hum. Genet 60, 505–514 (1997).
Gorski, B., Jakubowska, A., Huzarski, T., Byrski, T., Gronwald, J., Grzybowska, E. et al. A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int. J. Cancer 110, 683–686 (2004).
Dobričić, J., Branković-Magić, M., Filipović, S. & Radulović, S. Novel BRCA1/2 mutations in Serbian breast and breast-ovarian cancer patients with hereditary predisposition. Cancer Genet. Cytogenet. 202, 27–32 (2010).
Papp, J., Raicevic, L., Milasin, J., Dimitrijevic, B., Radulovic, S. & Olah, E. Germline mutation analysis of BRCA1 and BRCA2 genes in Yugoslav breast-ovarian cancer families. Oncol. Rep. 6, 1435–1438 (1999).
Konstantopoulou, I., Janković, R., Raičević, L., Ladopoulou, A., Armaou, S., Nikolopoulos, G. et al. BRCA1 and BRCA2 genes mutation analysis in patients with a family history of breast and ovarian cancer. Jugoslav. Med. Biochem. 23, 271–277 (2004).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Levanat, S., Musani, V., Cvok, M. L., Susac, I., Sabol, M., Ozretic, P. et al. Three novel BRCA1/BRCA2 mutations in breast/ovarian cancer families in Croatia. Gene 498, 169–176 (2012).
Davies, A. A., Masson, J. Y., McIlwraith, M. J., Stasiak, A. Z., Stasiak, A., Venkitaraman, A. R. et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell. 7, 273–282 (2001).
Haraldsson, K., Loman, N., Zhang, Q. X., Johannsson, O., Olsson, H. & Borg, A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 58, 1367–1371 (1998).
Claes, K., Poppe, B., Machackova, E., Coene, I., Foretova, L., De Paepe, A. et al. Differentiating pathogenic mutations from polymorphic alterations in the splice sites of BRCA1 and BRCA2. Genes Chromosomes Cancer 37, 314–320 (2003).
Mazoyer, S., Dunning, A. M., Serova, O., Dearden, J., Puget, N., Healey, C. S. et al. A polymorphic stop codon in BRCA2. Nat. Genet. 14, 253–254 (1996).
Wu, K., Hinson, S. R., Ohashi, A., Farrugia, D., Wendt, P., Tavtigian, S. V. et al. Functional evaluation and cancer risk assessment of BRCA2 unclassified variants. Cancer Res. 65, 417–426 (2005).
Martin, S. T., Matsubayashi, H., Rogers, C. D., Philips, J., Couch, F. J., Brune, K. et al. Increased prevalence of the BRCA2 polymorphic stop codon K3326X among individuals with familial pancreatic cancer. Oncogene 24, 3652–3656 (2005).
Abkevich, V., Zharkikh, A., Deffenbaugh, A. M., Frank, D., Chen, Y., Shattuck, D. et al. Analysis of missense variation in human BRCA1 in the context of interspecific sequence variation. J. Med. Genet. 41, 492–507 (2004).
Arnold, N., Peper, H., Bandick, K., Kreikemeier, M., Karow, D., Teegen, B. et al. Establishing a control population to screen for the occurrence of nineteen unclassified variants in the BRCA1 gene by denaturing high-performance liquid chromatography. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 782, 99–104 (2002).
Mirkovic, N., Marti-Renom, M. A., Weber, B. L., Sali, A. & Monteiro, A. N. Structure-based assessment of missense mutations in human BRCA1: implications for breast and ovarian cancer predisposition. Cancer Res. 64, 3790–3797 (2004).
Phelan, C. M., Đapić, V., Tice, B., Favis, R., Kwan, E., Barany, F. et al. Classification of BRCA1 missense variants of unknown clinical significance. J. Med. Genet. 42, 138–146 (2005).
Janezic, S. A., Ziogas, A., Krumroy, L. M., Krasner, M., Plummer, S. J., Cohen, P. et al. Germline BRCA1 alterations in a population-based series of ovarian cancer cases. Hum. Mol. Genet. 8, 889–897 (1999).
Dunning, A. M., Chiano, M., Smith, N. R., Dearden, J., Gore, M., Oakes, S. et al. Common BRCA1 variants and susceptibility to breast and ovarian cancer in the general population. Hum. Mol. Genet. 6, 285–289 (1997).
Seymour, I. J., Casadei, S., Zampiga, V., Rosato, S., Danesi, R., Falcini, F. et al. Disease family history and modification of breast cancer risk in common BRCA2 variants. Oncol. Rep. 19, 783–786 (2008).
Wenham, R. M., Schildkraut, J. M., McLean, K., Calingaert, B., Bentley, R. C., Marks, J. et al. Polymorphisms in BRCA1 and BRCA2 and risk of epithelial ovarian cancer. Clin. Cancer Res. 9, 4396–4403 (2003).
Couch, F. J., Farid, L. M., Deshano, M. L., Tavtigian, S. V., Calzone, K., Campeau, L. et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat. Genet. 13, 123–125 (1996).
Greenman, J., Mohammed, S., Ellis, D., Watts, S., Scott, G., Izatt, L. et al. Identification of missense and truncating mutations in the BRCA1 gene in sporadic and familial breast and ovarian cancer. Genes Chromosomes Cancer 21, 244–249 (1998).
Goldgar, D. E., Easton, D. F., Deffenbaugh, A. M., Monteiro, A. N., Tavtigian, S. V., Couch, F. J. et al. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am. J. Hum. Genet. 75, 535–544 (2004).
Petersen, G. M., Parmigiani, G. & Thomas, D. Missense mutations in disease genes: a Bayesian approach to evaluate causality. Am. J. Hum. Genet. 62, 1516–1524 (1998).
Fleming, M. A., Potter, J. D., Ramirez, C. J., Ostrander, G. K. & Ostrander, E. A. Understanding missense mutations in the BRCA1 gene: an evolutionary approach. Proc. Natl Acad. Sci. USA 100, 1151–1156 (2003).
Lee, T. C., Lee, A. S. & Li, K. B. Incorporating the amino acid properties to predict the significance of missense mutations. Amino Acids 35, 615–626 (2008).
Hadjisavvas, A., Adamou, A., Kitsios, P., Phanis, C., Kyriacou, K. & Christodoulou, C. G. Q356R and S151RI are BRCA1 variants that may be associated with breast cancer in a Cypriot family. Oncol. Rep. 9, 383–386 (2002).
Healey, C. S., Dunning, A. M., Teare, M. D., Chase, D., Parker, L., Burn, J. et al. A common variant in BRCA2 is associated with both breast cancer risk and prenatal viability. Nat. Genet. 26, 362–364 (2000).
Spurdle, A. B., Hopper, J. L., Chen, X., Dite, G. S., Cui, J., McCredie, M. R. et al. The BRCA2 372 HH genotype is associated with risk of breast cancer in Australian women under age 60 years. Cancer Epidemiol. Biomarkers Prev. 11, 413–416 (2002).
The Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the breast cancer association consortium. J. Natl Cancer Inst. 98, 1382–1396 (2006).
Fuks, F., Milner, J. & Kouzarides, T. BRCA2 associates with acetyltransferase activity when bound to P/CAF. Oncogene 17, 2351–2354 (1998).
Elit, L. Familial ovarian cancer. Can. Fam. Physician 47, 778–784 (2001).
Finch, A., Beiner, M., Lubinski, J., Lynch, H. T., Moller, P., Rosen, B. et alHereditary Ovarian Cancer Clinical Study Group Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 296, 185–192 (2006).
The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl Cancer Inst. 91, 1310–1316 (1999).
Loman, N., Johannsson, O., Kristoffersson, U., Olsson, H. & Borg, A. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J. Natl Cancer Inst. 93, 1215–1223 (2001).
Fackenthal, J. D. & Olopade, O. I. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat. Rev. Cancer 7, 937–948 (2007).
Metcalfe, K., Lynch, H. T., Ghadirian, P., Tung, N., Olivotto, I., Warner, E. et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 22, 2328–2335 (2004).
Acknowledgements
This study was supported by a grant of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant number 41026). We thank Professor Dr Zvonko Magic and his co-workers on their permanent support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dobričić, J., Krivokuća, A., Brotto, K. et al. Serbian high-risk families: extensive results on BRCA mutation spectra and frequency. J Hum Genet 58, 501–507 (2013). https://doi.org/10.1038/jhg.2013.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2013.30
Keywords
This article is cited by
-
BRCA1 and BRCA2 germline variants in breast cancer patients from the Republic of Macedonia
Breast Cancer Research and Treatment (2018)
-
Prevalence and Penetrance of BRCA1 and BRCA2 Germline Mutations in Colombian Breast Cancer Patients
Scientific Reports (2017)
-
Spectrum and frequencies of BRCA1/2 mutations in Bulgarian high risk breast cancer patients
BMC Cancer (2015)