Abstract
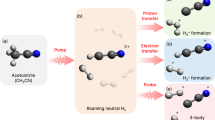
‘Roaming’ is a new and unusual class of reaction mechanism that has recently been discovered in unimolecular dissociation reactions of isolated molecules in the gas phase. It is characterized by frustrated bond cleavage, after which the two incipient fragments ‘roam’ on a flat region of the potential energy surface before reacting with one another. Here, we provide evidence that supports roaming in the liquid phase. We are now able to explain previous solution-phase experiments by comparing them with new ultrafast transient absorption data showing the photoisomerization of gas-phase CHBr3. We see that, upon S0–S1 excitation, gas-phase CHBr3 isomerizes within 100 fs into the BrHCBr–Br species, which is identical to what has been observed in solution. Similar sub-100 fs isomerization is now also observed for BBr3 and PBr3 in solution upon S1 excitation. Quantum chemical simulations of XBr3 (X = B, P or CH) suggest that photochemical reactivity in all three cases studied is governed by S1/S0 conical intersections and can best be described as occurring through roaming-mediated pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Townsend, D. et al. The roaming atom: straying from the reaction path in formaldehyde. Science 306, 1158–1161 (2004).
Suits, A. G. Roaming atoms and radicals: a new mechanism in molecular dissociation. Acc. Chem. Res. 41, 873–881 (2008).
Herath, N. & Suits, A. G. Roaming radical reactions. J. Phys. Chem. Lett. 2, 642–647 (2011).
Bowman, J. M. & Sheler, B. C. Roaming radicals. Annu. Rev. Phys. Chem. 62, 531–553 (2011).
Houston, P. L. & Kable, S. H. Photodissociation of acetaldehyde as a second example of the roaming mechanism. Proc. Natl Acad. Sci. USA 103, 16079–16082 (2006).
Heazlewood, B. R. et al. Roaming is the dominant mechanism for molecular products in the acetaldehyde photodissociation. Proc. Natl Acad. Sci. USA 105, 12719–12724 (2008).
Hause, M. L., Herath, N., Zhu, R., Lin, M. C. & Suits, A. G. Roaming-mediated isomerization in the photodissociation of nitrobenzene. Nature Chem. 3, 932–937 (2011).
Harding, L. B. & Klippenstein, S. J. Roaming radical pathways for the decomposition of alkanes. J. Phys. Chem. Lett. 1, 3016–3020 (2010).
Grubb, M. P. et al. No straight path: roaming in both ground and excited state photolytic channels of NO3 → NO+O2 . Science 335, 1075–1077 (2012).
Grubb, M., Warter, M., Suits, A. & North, S. W. Evidence of roaming dynamics and multiple channels for molecular elimination in NO3 photolysis. J. Phys. Chem. Lett. 1, 2455–2458 (2010).
Xiao, H., Maeda, S. & Morokuma, K. Excited-state roaming dynamics in photolysis of a nitrate radical. J. Phys. Chem. Lett. 2, 934–938 (2011).
Pal, S. K., Mereshchenko, A. S., Butaeva, E. V., El-Khoury, P. Z. & Tarnovsky, A. N. Global sampling of the photochemical reaction paths of bromoform by ultrafast deep-UV through near-IR transient absorption and ab initio multiconfigurational calculations. J. Chem. Phys. 138, 124501 (2013).
Cameron, M. & Bacskay, G. B. Stabilities, excitation energies, and dissociation reactions of CF2Cl2 and CF2Br2: quantum chemical computations of heats of formation of fluorinated methanes, methyls, and carbenes. J. Phys. Chem. A 104, 11212–11219 (2000).
El-Khoury, P. Z. et al. Characterization of iso-CF2I2 in frequency and ultrafast time domains. J. Chem. Phys. 132, 124501 (2010).
George, L. et al. Spectroscopic and computational studies of matrix-isolated iso-CHBr3: structure, properties, and photochemistry of iso-bromoform. J. Chem. Phys. 135, 124503 (2011).
Kalume, A., George, L. & Reid, S. A. Isomerization as a key path to molecular products in the gas-phase decomposition of halons. J. Phys. Chem. Lett. 1, 3090–3095 (2010).
Granovsky, A. A. Extended multi-configuration quasi-degenerate perturbation theory: the new approach to multi-state multi-reference perturbation theory. J. Chem. Phys. 134, 214113 (2011).
Pulay, P. A Perspective on the CASPT2 method. Int. J. Quant. Comp. 111, 3273–3364 (2011).
Serrano-Andrés, L., Merchán, M. & Lindh, R. Computation of conical intersections by using perturbation techniques. J. Chem. Phys. 122, 104107 (2005).
Koch, H. & Jørgensen, P. Coupled cluster response functions. J. Chem. Phys. 93, 3333–3344 (1990).
Briggs, A. G. & Simmons, R. E. Photochemical reactions of some boron trihalides. Naturwissenschaften 67, 402–402 (1980).
Briggs, A. G. The flash photolysis of phosphorus tribromide and carbon oxysulphide. Spectrochim. Acta A 37, 457–458 (1981).
Bramwell, M. J., Jaeger, S. E. & Whitehead, J. C. The 193 nm photolysis of phosphorus trichloride and phosphorus tribromide. Chem. Phys. Lett. 196, 547–551 (1992).
Bramwell, M. J., Hughes, C., Jaeger, S. E. & Whitehead, J. C. The 248 nm photolysis of phosphorus trichloride and phosphorus tribromide. Chem. Phys. 183, 127–134 (1994).
Latifzadeh, L. & Balasubramanian, K. Electronic states of PBr2 and PBr2+. Chem. Phys. Lett. 258, 393–399 (1996).
Miller, J. H. & Andrews, L. Matrix photoionization and radiolysis of boron trihalides. Infrared and ultraviolet spectra of BC13+ and BBr3+ and infrared spectra of BC12 and BBr2 . J. Am. Chem. Soc. 102, 4900–4906 (1980).
Moroz, A. & Sweany, L. Photolysis of argon matrices containing tribromoboron and dihydrogen: synthesis of hydroborones via dibromoboron. Inorg. Chem. 31, 5236–5242 (1992).
Jan-Khan, M. & Samuel, R. Absorption spectra and photodissociation of some inorganic molecules. Proc. Phys. Soc. 48, 626–641 (1936).
Kennedy, T., Sinclair, R. S. & Sinclair, T. J. The U.V. spectrum and photolysis of phosphorus halides in hydrocarbon solvents. II Phosphorus tribromide and phosphorus pentabromide in cyclohexane. J. Inorg. Nucl. Chem. 33, 2369–2376 (1971).
Alfassi, Z. B., Huie, R. E., Mittal, J. P., Neta, P. & Shoute, L. C. T. Charge-transfer complexes of bromine atoms with haloalkanes and alkanes. J. Phys. Chem. 97, 9120–9123 (1993).
Harris, A. L., Berg, M. & Harris, C. B. Studies of chemical reactivity in the condensed phase. I. The dynamics of iodine photodissociation and recombination on a picosecond time scale and comparison to theories for chemical reactions in solution. J. Chem. Phys. 84, 788–806 (1986).
Kliner, D. A. V., Alfano, J. C. & Barbara, P. F. Photodissociation and vibrational relaxation of I2− in ethanol. J. Chem. Phys. 98, 5375–5389 (1993).
Dieter Bingemann, D., King, A. M. & Crim, F. F. Transient electronic absorption of vibrationally excited CH2I2: watching energy flow in solution. J. Chem. Phys. 113, 5018–5025 (2000).
Schwartz, B. J., King, J. C., Zhang, J. Z. & Harris, C. B. Direct femtosecond measurements of single collision dominated geminate recombination times of small molecules in liquids. Chem. Phys. Lett. 203, 503–508 (1993).
Benjamin, I. Photodissociation of ICN in liquid chloroform: molecular dynamics of ground and excited state recombination, cage escape, and hydrogen abstraction reaction. J. Chem. Phys. 103, 2459–2471 (1995).
Moscun, A. C. & Bradforth, S. E. Photodissociation of ICN in polar solvents: evidence for long lived rotational excitation in room temperature liquids. J. Chem. Phys. 119, 4500–4515 (2003).
Roos, B. O. The complete active space self-consistent field method and its applications in electronic structure calculations. Adv. Chem. Phys. 69, 399–445 (1987).
Clark, R. J. H. & Rippon, D. M. The vapor phase Raman spectra, Raman band contour analyses, Coriolis constants, force constants, and values for thermodynamic functions of the trihalides of group V. J. Mol. Spectrosc. 52, 58–71 (1974).
Anderson, A. & Lettress, L. M. Raman spectra of molecular crystals at high pressures: VIII – boron tribromide. J. Raman Spectrosc. 34, 684–687 (2003).
Purvis, G. D. III & Bartlett, R. J. A full coupled-cluster singles and doubles model—the inclusion of disconnected triples. J. Chem. Phys. 76, 1910–1918 (1982).
Elles, C. G. & Crim, F. F. Connecting chemical dynamics in gases and liquids. Annu. Rev. Phys. Chem. 57, 273–302 (2006).
Oliver, T. A. A., Zhang, Y., Ashfold, M. N. R. & Bradforth, S. E. Linking photochemistry in the gas and solution phases: S–H bond fission in p-methylthiophenol following UV excitation. Faraday Disc. 150, 439–458 (2011).
Fleming, G. R. Chemical Applications of Ultrafast Spectroscopy 126 (Oxford Univ. Press, 1986).
Kovalenko, S. A., Dobryakov, A. L., Ruthmann, J. & Ernsting, N. P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 59, 2369–2382 (1999).
Varga, Z., Kolonits, M. & Hargittai, M. Comprehensive study of the structure of aluminium trihalides from electron diffraction and computation. Struct. Chem. 23, 879–893 (2012).
Tully, J. C. Molecular dynamics with electronic transitions. J. Chem. Phys. 93, 1061–1071 (1990).
Acknowledgements
This work was supported by the National Science Foundation (CAREER CHE-0847707 and CHE-0923360). The authors acknowledge an allocation of computer time from the Ohio Supercomputer Center (PCS0204-7) and the Extreme Science and Engineering Discovery Environment (XSEDE, CHE130073). The authors thank R. M. Wilson and P.Z. El-Khoury for discussions. This work would not have been possible without discussions with M. Olivucci, as well as his guidance through the semiclassical trajectory calculations.
Author information
Authors and Affiliations
Contributions
A.S.M. conceived and designed the experiments, and carried them out together with E.V.B. and A.N.T. E.V.B. and V.A.B. designed and performed the high-level quantum chemical simulations. A.E. analysed the semiclassical trajectories of boron and phosphorus tribromides. A.N.T. analysed the data and supervised the project. The first three authors contributed equally to this work, and all authors contributed to writing sections of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8326 kb)
Supplementary information
Supplementary Movie 1 (AVI 600 kb)
Supplementary information
Supplementary Movie 2 (AVI 470 kb)
Supplementary information
Supplementary Movie 3 (AVI 505 kb)
Supplementary information
Supplementary data 1. The xyz coordinates of the BBr3 gas-phase CASPT2 semiclassical trajectory. (TXT 24 kb)
Supplementary information
Supplementary data 2. The xyz coordinates of the CHBr3 gas-phase CASPT2 semiclassical trajectory. (TXT 21 kb)
Supplementary information
Supplementary data 3. The xyz coordinates of the PBr3 gas-phase CASPT2 semiclassical trajectory. (TXT 24 kb)
Supplementary information
Supplementary data 4. The xyz-file describing the trajectory of bromoform in the acetonitrile solvent cage: CASSCF QM/MM molecular dynamics simulation of CHBr3 in a rigid CH3CN cavity. (TXT 32421 kb)
Rights and permissions
About this article
Cite this article
Mereshchenko, A., Butaeva, E., Borin, V. et al. Roaming-mediated ultrafast isomerization of geminal tri-bromides in the gas and liquid phases. Nature Chem 7, 562–568 (2015). https://doi.org/10.1038/nchem.2278
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nchem.2278
This article is cited by
-
Capturing the generation and structural transformations of molecular ions
Nature (2024)
-
Deep learning study of tyrosine reveals that roaming can lead to photodamage
Nature Chemistry (2022)