Abstract
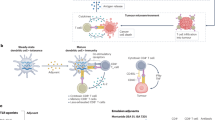
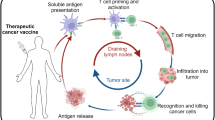
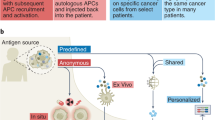
Therapeutic cancer vaccines are being developed with the intention of treating existing tumors or preventing tumor recurrence. While the results of clinical trials, predominantly in the metastatic setting have been sobering, the central hypothesis of active immunotherapy i.e. that the human immune system can be activated to recognize and destroy tumor cells, remains a viable one. We believe that a fundamental shift in how clinical trials are performed, and what concepts they test is required to make meaningful strides towards future clinical use of cancer vaccines. First, we must reappraise whether the metastatic setting is the appropriate arena to test these agents. Second, we must arrive at a consensus on the most important biologic endpoints and rapidly test vaccines for their ability to achieve these endpoints. Third, we need to expend more effort on understanding how to manipulate the immune system beyond the initial stimulation provided by a vaccine. Fourth, in order to permit comparison of results across different studies, it would be helpful to narrow down the large number of vaccine platforms. We will discuss the current state of development of cancer vaccines and the relevance for future clinical use of these agents to treat and prevent cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rosenberg SA et al. (2004) Reply to “Cancer vaccines: pessimism in check”. Nat Med 10: 1279–1280
Rosenberg SA et al. (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10: 909–915
Mocellin S et al. (2004) Correspondence 1: Cancer vaccines: pessimism in check. Nat Med 10: 1278–1279
Timmerman JM and Levy R (2004) Correspondence 2: Cancer vaccines: pessimism in check. Nat Med 10: 1279
Mayordomo J et al. (2004) Long-term follow-up of patients concomitantly treated with hormone therapy in a prospective controlled randomized multicenter clinical study comparing STn-KLH vaccine with KLH control in stage IV breast cancer following first-line chemotherapy. 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 22 (Suppl): [abstract 2603]
Schadendorf D et al. (2004) Dacarbacine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) as first-line treatment of patients with metastatic melanoma: Results of a prospective-randomized phase III study. ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 22 (Suppl): [abstract 7508]
Small EJ et al. (2003) A randomized, placebo-controlled phase III trial of APC8015 in patients with androgen-independent prostate cancer (AiPCa). Proc Am Soc Clin Oncol 22: 382 [abstract 1534]
Ridgway D (2003) The first 1000 dendritic cell vaccines. Cancer Invest 21: 876–886
Ribas A et al. (2003) Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol 21: 2415–2432
Mocellin S et al. (2004) Part II: Vaccines for haematological malignant disorders. Lancet Oncol 5: 727–737
Mocellin S et al. (2004) Part I: Vaccines for solid tumours. Lancet Oncol 5: 681–689
Jocham D et al. (2004) Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 363: 594–599
Bystryn JC et al. (2001) Double-blind trial of a polyvalent, shed-antigen, melanoma vaccine. Clin Cancer Res 7: 1882–1887
Sondak VK et al. (2002) Adjuvant immunotherapy of resected, intermediate-thickness node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol 20: 2058–2066
Sosman JA et al. (2002) Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol 20: 2067–2075
Sosman JA et al. (2002) HLA-A2 and/or HLA-C3 expression defines a subset of T3N0 melanoma patients with improved overall survival from melacine vaccine: An updated analysis of SWOG 9035. Proc Am Soc Clin Oncol 20: 340 [abstract 1359]
Bittner M et al. (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406: 536–540
Timmerman JM et al. (2002) Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood 99: 1517–1526
Weng W et al. (2004) Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol 22: 4665–4672
Novellino L et al. (2004) A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother [10.1007/s00262-004-0560-6]
Hickman HD et al. (2004) Toward a definition of self: proteomic evaluation of the class I peptide repertoire. J Immunol 172: 2944–2952
DiFronzo LA et al. (2002) Enhanced humoral immune response correlates with improved disease-free and overall survival in American Joint Committee on Cancer stage II melanoma patients receiving adjuvant polyvalent vaccine. J Clin Oncol 20: 3242–3248
Disis ML et al. (2004) Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol 24: 571–578
Vigneron N et al. (2004) An antigenic peptide produced by peptide splicing in the proteasome. Science 304: 587–590
Gilliam AD et al. (2004) Randomised, double blind, placebo-controlled, multi-centre, group-sequential trial of G17DT for patients with advanced pancreatic cancer unsuitable or unwilling to take chemotherapy. ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 22 (Suppl): [abstract 2511]
Shomura H et al. (2004) Identification of epidermal growth factor receptor-derived peptides immunogenic for HLA-A2(+) cancer patients. Br J Cancer 90: 1563–1571
Niethammer AG et al. (2002) A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 8: 1369–1375
Slingluff CL Jr et al. (2003) Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol 21: 4016–4026
Kaufman HL et al. (2004) Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 22: 2122–2132
Disis ML et al. (2004) Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein-based vaccine. J Clin Oncol 22: 1916–1925
Vieweg J et al. (2004) Enhancement of antitumor immunity following depletion of CD4+CD25+ regulatory T cells. 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 22 (Suppl): [abstract 2506]
Gregor PD et al. (2004) CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine 22: 1700–1708
Pockaj BA et al. (2004) Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol 11: 328–339
Zeytin HE et al. (2004) Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res 64: 3668–3678
Eralp Y et al. (2004) Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res 6: R275–R283
Wolpoe ME et al. (2003) HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol 171: 2161–2169
Mazzaferro V et al. (2003) Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res 9: 3235–3245
Slingluff CL Jr et al. (2004) Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol 22: 4474–4485
Khan AN et al. (2004) An epigenetically altered tumor cell vaccine. Cancer Immunol Immunother 53: 748–754
Hodge JW et al. (2003) Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res 9: 1837–1849
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- GM-CSF
-
Granulocyte-macrophage colony-stimulating factor
- Id (IDIOTYPE)
-
A specific protein antigen made by B lyphocyte cells, which distinguishes a clone of immunoglobulin-producing cells from other clones
- ELISPOT ASSAY
-
Enzyme-linked immunospot assay is a highly sensitive tool for analyzing immunological secretions of peripheral blood cell populations
Rights and permissions
About this article
Cite this article
Morse, M., Chui, S., Hobeika, A. et al. Recent developments in therapeutic cancer vaccines. Nat Rev Clin Oncol 2, 108–113 (2005). https://doi.org/10.1038/ncponc0098
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/ncponc0098
This article is cited by
-
CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients
Scientific Reports (2016)
-
Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer
Breast Cancer Research and Treatment (2014)
-
In vivo imaging of therapy-induced anti-cancer immune responses in humans
Cellular and Molecular Life Sciences (2013)
-
Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer
Cancer Immunology, Immunotherapy (2011)
-
Synergistic antitumor effects of CpG oligodeoxynucleotide and STAT3 inhibitory agent JSI‐124 in a mouse melanoma tumor model
Immunology & Cell Biology (2008)