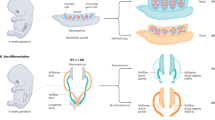
Abstract
Sex differentiation in mammals occurs in three steps. The first is the establishment of chromosomal sex at fertilization, followed by the differentiation of the gonad into an ovary or testis, and finally the establishment of the phenotypic sex of the embryo and adult, which is regulated by the gonad. Disruption of any of these stages gives rise to sexual ambiguities that include 46,XY pure gonadal dysgenesis, 46,XX true hermaphroditism, and variable degrees of intersexuality. In this review, we focus on the development of the mammalian gonad from a bipotential primordium that differentiates into either an ovary or a testis. We describe the recent increase in our knowledge of the genetic defects that directly affect gonadal development, sex determination, and sex differentiation, with emphasis on the comparison of genetic studies in mice with studies of naturally occurring mutations in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Swain A and Lovell-Badge R (1999) Mammalian sex determination: a molecular drama. Genes Dev 13: 755–767
Swain A and Lovell-Badge R (2002) Sex determination and differentiation. In Mouse Development: Patterning, Morphogenesis and Organogenesis, 371–393 (Eds Rossant J and Tam PP) London: Academic Press Ltd
Brennan J and Capel B (2004) One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5: 509–521
Yao HH (2005) The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 230: 87–93
McLaren A (1991) Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays 13: 151–156
Val P et al. (2003) SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1: 8
Luo X et al. (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77: 481–490
Bland ML et al. (2004) Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18: 941–952
Mallet D et al. (2004) Gonadal dysgenesis without adrenal insufficiency in a 46,XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab 89: 4829–4832
Hasegawa T et al. (2004) Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab 89: 5930–5935
Bland ML et al. (2000) Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97: 14488–14493
Hastie ND (2001) Life, sex, and WT1 isoforms—three amino acids can make all the difference. Cell 106: 391–394
Reddy JC and Licht JD (1996) The WT1 Wilms' tumor suppressor gene: how much do we really know? Biochim Biophys Acta 1287: 1–28
Hammes A et al. (2001) Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106: 319–329
Jaubert F et al. (2003) Gonad development in Drash and Frasier syndromes depends on WT1 mutations. Arkh Patol 65: 40–44
Katoh-Fukui Y et al. (1998) Male-to-female sex reversal in M33 mutant mice. Nature 393: 688–692
Miyamoto N et al. (1997) Defects of urogenital development in mice lacking Emx2. Development 124: 1653–1664
Birk OS et al. (2000) The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403: 909–913
Haqq CM and Donahoe PK (1998) Regulation of sexual dimorphism in mammals. Physiol Rev 78: 1–33
Klamt B et al. (1998) Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/− KTS splice isoforms. Hum Mol Genet 7: 709–714
Viger RS et al. (1998) Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the müllerian inhibiting substance promoter. Development 125: 2665–2675
Tevosian SG et al. (2002) Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129: 4627–4634
Tremblay JJ and Viger RS (1999) Transcription factor GATA-4 enhances müllerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol 13: 1388–1401
Tremblay JJ and Viger RS (2001) GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology 142: 977–986
Canning CA and Lovell-Badge R (2002) Sry and sex determination: how lazy can it be? Trends Genet 18: 111–113
Mansour S et al. (1995) A clinical and genetic study of campomelic dysplasia. J Med Genet 32: 415–420
Huang BL et al. (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87: 349–353
Bishop CE et al. (2000) A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26: 490–494
Vidal V et al. (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28: 216–217
Chaboissier MC et al. (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131: 1891–1901
Arango NA et al. (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99: 409–419
Schmahl J and Capel B (2003) Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol 258: 264–276
Colvin JS et al. (2001) Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104: 875–889
Nef S et al. (2003) Testis determination requires insulin receptor family function in mice. Nature 426: 291–295
Nachtigal MW et al. (1998) Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93: 445–454
Zanaria E et al. (1994) An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372: 635–641
Muscatelli F et al. (1994) Mutations in the DAX1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372: 672–676
Bardoni B et al. (1994) A dosage-sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet 7: 497–501
Swain A et al. (1998) Dax1 antagonizes Sry action in mammalian sex determination. Nature 391: 761–767
Bouma GJ et al. (2005) Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development 132: 3045–3054
Meeks JJ et al. (2003) Dax1 is required for testis determination. Nat Genet 34: 32–33
Lalli E and Sassone-Corsi P (2003) DAX1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol 17: 1445–1453
Achermann JC et al. (2001) Phenotypic spectrum of mutations in DAX1 and SF-1. Mol Cell Endocrinol 185: 17–25
Tabarin A et al. (2000) A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest 105: 321–328
Ozisik G et al. (2003) An alternate translation initiation site circumvents an amino-terminal DAX1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab 88: 417–423
Yao HH et al. (2002) Desert hedgehog/patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16: 1433–1440
Clark AM et al. (2000) Desert hedgehog (Dhh) gene is required in the mouse for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63: 1825–1838
Umehara F et al. (2000) A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet 67: 1302–1305
Brennan J et al. (2003) Pdgfr-a mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17: 800–810
Kitamura K et al. (2002) Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet 32: 359–369
Jeyasuria P et al. (2004) Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18: 1610–1619
Tamura M et al. (2001) Pod-1/capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev 102: 135–144
Cui S et al. (2004) Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131: 4095–4105
Crisponi L et al. (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus/inversus syndrome. Nat Genet 27: 159–166
Crisponi L et al. (2004) FOXL2 inactivation by a translocation 171 kb away: analysis of 500 kb of chromosome 3 for candidate long-range regulatory sequences. Genomics 83: 757–764
Uda M et al. (2004) Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13: 1171–1181
Schmidt D et al. (2004) The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131: 933–942
Pailhoux E et al. (2001) A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet 29: 453–458
Pannetier M et al. (2005) Ovarian-specific expression of a new gene regulated by the goat PIS region and transcribed by a FOXL2 bidirectional promoter. Genomics 85: 715–726
Pailhoux E et al. (2002) Ontogenesis of female-to-male sex-reversal in XX polled goats. Dev Dyn 224: 39–50
Vainio S et al. (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405–409
Jeays-Ward C et al. (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130: 3663–3670
Biason-Lauber A et al. (2004) A WNT4 mutation associated with müllerian duct regression and virilization in a 46,XX woman. N Engl J Med 351: 792–798
Jeays-Ward C et al. (2004) Wnt4 is required for proper male as well as female sexual development. Dev Biol 276: 431–440
Jordan BK et al. (2001) Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68: 1102–1109
Jordan BK et al. (2003) Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/b-catenin synergy. Proc Natl Acad Sci USA 100: 10866–10871
Yao HH et al. (2004) Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn 230: 210–215
Ottolenghi C et al. (2001) Absence of mutations involving the LIM homeobox domain gene LHX9 in 46,XY gonadal agenesis and dysgenesis. J Clin Endocrinol Metab 86: 2465–2469
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Val, P., Swain, A. Mechanisms of Disease: normal and abnormal gonadal development and sex determination in mammals. Nat Rev Urol 2, 616–627 (2005). https://doi.org/10.1038/ncpuro0354
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/ncpuro0354