Abstract
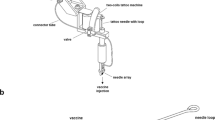
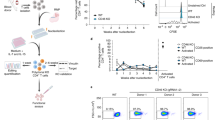
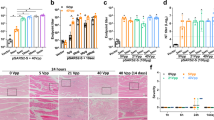
DNA vaccines have many potential benefits but have failed to generate robust immune responses in humans. Recently, methods such as in vivo electroporation have demonstrated improved performance, but an optimal strategy for safe, reproducible, and pain-free DNA vaccination remains elusive. Here we report an approach for rapid implantation of vaccine-loaded polymer films carrying DNA, immune-stimulatory RNA, and biodegradable polycations into the immune-cell-rich epidermis, using microneedles coated with releasable polyelectrolyte multilayers. Films transferred into the skin following brief microneedle application promoted local transfection and controlled the persistence of DNA and adjuvants in the skin from days to weeks, with kinetics determined by the film composition. These ‘multilayer tattoo’ DNA vaccines induced immune responses against a model HIV antigen comparable to electroporation in mice, enhanced memory T-cell generation, and elicited 140-fold higher gene expression in non-human primate skin than intradermal DNA injection, indicating the potential of this strategy for enhancing DNA vaccination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kutzler, M. A. & Weiner, D. B. DNA vaccines: Ready for prime time? Nature Rev. Genet. 9, 776–788 (2008).
Rice, J., Ottensmeier, C. H. & Stevenson, F. K. DNA vaccines: Precision tools for activating effective immunity against cancer. Nature Rev. Cancer 8, 108–120 (2008).
Ulmer, J. B., Wahren, B. & Liu, M. A. Gene-based vaccines: Recent technical and clinical advances. Trends Mol. Med. 12, 216–222 (2006).
Sardesai, N. Y. & Weiner, D. B. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 23, 421–429 (2011).
Weir, E. & Hatch, K. Preventing cold chain failure: Vaccine storage and handling. Can. Med. Assoc. J. 171, 1050 (2004).
Rapiti, E., Pruss-Ustun, A. & Hutin, Y. WHO Environmental Burden of Disease Series (World Health Organization, 2003).
Dicko, M. et al. Safety of immunization injections in Africa: Not simply a problem of logistics. Bull. World Health Org. 78, 163–169 (2000).
Giudice, E. L. & Campbell, J. D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 58, 68–89 (2006).
Bins, A. D. et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nature Med. 11, 899–904 (2005).
Johansen, P. et al. Antigen kinetics determines immune reactivity. Proc. Natl Acad. Sci. USA 105, 5189–5194 (2008).
Jewell, C. M., Bustamante, L. S. C. & Irvine, D. J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011).
Prlic, M., Hernandez-Hoyos, G. & Bevan, M. J. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 203, 2135–2143 (2006).
Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 277, 1232–1237 (1997).
Hammond, P. T. Engineering materials layer-by-layer: Challenges and opportunities in multilayer assembly. AIChE J. 57, 2928–2940 (2011).
Macdonald, M., Rodriguez, N. M., Smith, R. & Hammond, P. T. Release of a model protein from biodegradable self assembled films for surface delivery applications. J. Controlled Release 131, 228–234 (2008).
MacDonald, M. L. et al. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 32, 1446–1453 (2011).
Shah, N. J. et al. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 32, 6183–6193 (2011).
Doh, J. & Irvine, D. J. Photogenerated polyelectrolyte bilayers from an aqueous-processible photoresist for multicomponent protein patterning. J. Am. Chem. Soc. 126, 9170–9171 (2004).
DeMuth, P. C., Su, X., Samuel, R. E., Hammond, P. T. & Irvine, D. J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv. Mater. 22, 4851–4856 (2010).
Krogman, K. C., Lowery, J. L., Zacharia, N. S., Rutledge, G. C. & Hammond, P. T. Spraying asymmetry into functional membranes layer-by-layer. Nature Mater. 8, 512–518 (2009).
Jewell, C. M., Zhang, J., Fredin, N. J. & Lynn, D. M. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J. Controlled Release 106, 214–223 (2005).
Katz, J. S., Doh, J. & Irvine, D. J. Composition-tunable properties of amphiphilic comb copolymers containing protected methacrylic acid groups for multicomponent protein patterning. Langmuir 22, 353–359 (2006).
Little, S. R. et al. Poly- β amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl Acad. Sci. USA 101, 9534–9539 (2004).
Lynn, D. M. & Langer, R. Degradable poly(β-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 122, 10761–10768 (2000).
Su, X., Kim, B-S., Kim Sara, R., Hammond Paula, T. & Irvine Darrell, J. Layer-by-layer-assembled multilayer films for transcutaneous drug and vaccine delivery. ACS Nano 3, 3719–3729 (2009).
Boes, M. et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418, 983–988 (2002).
Jewell, C. M. et al. Release of plasmid DNA from intravascular stents coated with ultrathin multilayered polyelectrolyte films. Biomacromolecules 7, 2483–2491 (2006).
Zhang, J., Fredin, N. J., Janz, J. F., Sun, B. & Lynn, D. M. Structure/property relationships in erodible multilayered films: Influence of polycation structure on erosion profiles and the release of anionic polyelectrolytes. Langmuir 22, 239–245 (2006).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nature Med. 15, 455–461 (2009).
Soria-Castro, I. et al. Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J. Biol. Chem. 285, 33805–33815 (2010).
Bechler, S. L. & Lynn, D. M. Characterization of degradable polyelectrolyte multilayers fabricated using DNA and a fluorescently-labeled poly(β-amino ester): Shedding light on the role of the cationic polymer in promoting surface-mediated gene delivery. Biomacromolecules 13, 542–552 (2012).
Elnekave, M., Furmanov, K. & Hovav, A-H. Intradermal naked plasmid DNA immunization: Mechanisms of action. Expert Rev. Vaccines 10, 1169–1182 (2011).
Van Drunen Littel-van den Hurk, S. & Hannaman, D. Electroporation for DNA immunization: Clinical application. Expert Rev. Vaccines 9, 503–517 (2010).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Greenland, J. R. et al. β-amino ester polymers facilitate in vivo DNA transfection and adjuvant plasmid DNA immunization. Mol. Ther. 12, 164–170 (2005).
Saade, F. & Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 11, 189–209 (2012).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nature Med. 16, 915–920 (2009).
Zhu, Q. et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl Acad. Sci. USA 106, 7968–7973 (2009).
Chen, X. et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Controlled Release 148, 327–333 (2010).
Gill, H. S., Soederholm, J., Prausnitz, M. R. & Saellberg, M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 17, 811–814 (2010).
Mikszta, J. A. et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nature Med. 8, 415–419 (2002).
Song, J-M. et al. DNA vaccination in the skin using microneedles improves protection against influenza. Mol. Ther. 20, 1472–1480 (2012).
Verstrepen, B. E. et al. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine 26, 3346–3351 (2008).
Denet, A-R., Vanbever, R. & Preat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 56, 659–674 (2004).
Wallace, M. et al. Tolerability of two sequential electroporation treatments using MedPulser DNA Delivery System (DDS) in healthy adults. Mol. Ther. 17, 922–928 (2009).
Boudou, T., Crouzier, T., Ren, K., Blin, G. & Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 22, 441–467 (2010).
Dimitrova, M. et al. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc. Natl Acad. Sci. USA 105, 16320–16325 (2008).
Jessel, N. et al. Multiple and time-scheduled in situ DNA delivery mediated by -cyclodextrin embedded in a polyelectrolyte multilayer. Proc. Natl Acad. Sci. USA 103, 8618–8621 (2006).
Kim, B-S., Park, S. W. & Hammond, P. T. Hydrogen-bonding layer-by-layer-assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. ACS Nano 2, 386–392 (2008).
Kim, B-S., Smith, R. C., Poon, Z. & Hammond, P. T. MAD (multiagent delivery) nanolayer: Delivering multiple therapeutics from hierarchically assembled surface coatings. Langmuir 25, 14086–14092 (2009).
Lynn, D. M. Peeling back the layers: Controlled erosion and triggered disassembly of multilayered polyelectrolyte thin films. Adv. Mater. 19, 4118–4130 (2007).
Akinc, A., Anderson, D. G., Lynn, D. M. & Langer, R. Synthesis of poly(β-amino ester)s optimized for highly effective gene delivery. Bioconjug. Chem. 14, 979–988 (2003).
Stoitzner, P. et al. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: Augmentation by TNF- α and IL- 1β. J. Leukoc. Biol. 66, 462–470 (1999).
Acknowledgements
This work was supported in part by the Ragon Institute of MGH, MIT, and Harvard, the NIH (AI095109), and the Institute for Soldier Nanotechnology (Dept. of Defense contracts W911NF-07-D-0004 and W911NF-07-0004, T.O. 8). We thank R. Parkhill and M. Nguyen (VaxDesign) for assistance with microneedle array fabrication. D.J.I. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
P.C.D., P.T.H. and D.J.I. designed the experiments. P.C.D., D.H.B. and D.J.I. designed macaque skin studies. P.C.D. carried out the experiments; Y.M. performed in vitro nucleic acid release studies. B.H. synthesized the PNMP polymers. A.D.M. and J.A.K. collected the macaque skin. P.C.D., P.T.H. and D.J.I. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1176 kb)
Rights and permissions
About this article
Cite this article
DeMuth, P., Min, Y., Huang, B. et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nature Mater 12, 367–376 (2013). https://doi.org/10.1038/nmat3550
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nmat3550
This article is cited by
-
Hard polymeric porous microneedles on stretchable substrate for transdermal drug delivery
Scientific Reports (2022)
-
Microneedle-based insulin transdermal delivery system: current status and translation challenges
Drug Delivery and Translational Research (2022)
-
Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health
Nature Nanotechnology (2021)
-
Development of vaccine formulations: past, present, and future
Drug Delivery and Translational Research (2021)
-
Rapidly separable microneedle patch for the sustained release of a contraceptive
Nature Biomedical Engineering (2019)