Abstract
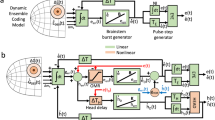
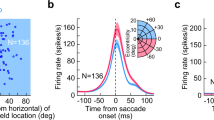
The superior colliculus (SC) is important for generating coordinated eye–head gaze saccades. Its deeper layers contain a retinotopically organized motor map in which each site is thought to encode a specific gaze saccade vector. Here we show that this fundamental assumption in current models of collicular function does not hold true during horizontal multi-step gaze shifts in darkness that are directed to a goal and composed of a sequence of gaze saccades separated by periods of steady fixation. At the start of a multi-step gaze shift in cats, neural activity on the SC's map was located caudally to encode the overall amplitude of the gaze displacement, not the first saccade in the sequence. As the gaze shift progressed, the locus of activity moved to encode the error between the goal and the current gaze position. Contrary to common belief, the locus of activity never encoded gaze saccade amplitude, except for the last saccade in the sequence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Moschovakis, A.K., Scudder, C.A. & Highstein, S.M. The microscopic anatomy and physiology of the mammalian saccadic system. Prog. Neurobiol. 50, 133–254 (1996).
Scudder, C.A., Kaneko, C.S. & Fuchs, A.F. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp. Brain Res. 142, 439–462 (2002).
Freedman, E.G., Stanford, T.R. & Sparks, D.L. Combined eye head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J. Neurophysiol. 76, 927–952 (1996).
Paré, M., Crommelinck, M. & Guitton, D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp. Brain Res. 101, 123–139 (1994).
Roucoux, A., Guitton, D. & Crommelinck, M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp. Brain Res. 39, 75–85 (1980).
Munoz, D.P. & Guitton, D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. II. Sustained discharges during motor preparation and fixation. J. Neurophysiol. 66, 1624–1641 (1991).
Munoz, D.P., Guitton, D. & Pelisson, D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J. Neurophysiol. 66, 1642–1666 (1991).
Freedman, E.G. & Sparks, D.L. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J. Neurophysiol. 78, 1669–1690 (1997).
Aizawa, H. & Wurtz, R.H. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J. Neurophysiol. 79, 2082–2096 (1998).
Quaia, C., Aizawa, H., Optican, L.M. & Wurtz, R.H. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J. Neurophysiol. 79, 2097–2110 (1998).
Lee, C., Rohrer, W.H. & Sparks, D.L. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332, 357–360 (1988).
Bergeron, A. & Guitton, D. Fixation neurons in superior colliculus encode gaze position error, the distance between current and desired gaze positions. Nat. Neurosci. 3, 932–939 (2000).
Bergeron, A. & Guitton, D. The superior colliculus and its control of fixation behavior via projections to brainstem omnipause neurons. Progr. Brain Res. 134, 97–107 (2001).
Bergeron, A. & Guitton, D. In multiple-step gaze shifts: omnipause (OPNs) and collicular fixation neurons encode gaze position error; OPNs gate saccades. J. Neurophysiol. 88, 1726–1742 (2002).
Munoz, D.P. & Guitton, D. Fixation and orientation control by the tecto-reticulo-spinal system in the cat whose head is unrestrained. Revue Neurol. (Paris) 145, 567–579 (1989).
Munoz, D.P. & Wurtz, R.H. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J. Neurophysiol. 70, 559–575 (1993).
Peck, C.K.A. Visual responses of neurones in cat superior colliculus in relation to fixation of targets. J. Physiol. 414, 301–315 (1989).
Peck, C.K. & Baro, J.A. Discharge patterns of neurons in the rostral superior colliculus of cat: activity related to fixation of visual and auditory targets. Exp. Brain Res. 113, 291–302 (1997).
Munoz, D.P. & Istvan, P.J. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J. Neurophysiol. 79, 1193–1209 (1998).
Krauzlis, R.J., Basso, M.A. & Wurtz, R.H. Shared motor error for multiple eye movements. Science 276, 1693–1695 (1997).
Krauzlis, R.J., Basso, M.A. & Wurtz, R.H. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth pursuit eye movements. J. Neurophysiol. 84, 876–891 (2000).
Guitton, D., Douglas, R.M. & Volle, M. Coordinated eye head movements in the cat. J. Neurophysiol. 52, 1030–1050 (1984).
Guitton, D. & Munoz, D.P. Control of orienting gaze shifts by the tecto reticulo spinal system in the heat-free cat. I. Identification, localization and effects of behavior on sensory responses. J. Neurophysiol. 66, 1605–1623 (1991).
Edelman, J.A. & Goldberg, M.E. Dependence of saccade-related activity in the primate superior colliculus on visual target presence. J. Neurophysiol. 86, 676–691 (2001).
Goossens, H.H. & Van Opstal, A.J. Blink-perturbed saccades in monkey. II. Superior colliculus activity. J. Neurophysiol. 83, 3430–3452 (2000).
Anderson, R.W., Keller, E.L., Gandhi, N.J. & Das, S. Two dimensional saccade related population activity in superior colliculus in monkey. J. Neurophysiol. 80, 798–817 (1998).
Gandhi, N.J. & Keller, E.L. Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region. J. Neurophysiol. 82, 3236–3253 (1999).
Munoz, D.P., Pelisson, D. & Guitton, D. Movement of neural activity on the superior colliculus motor map during gaze shifts. Science 251, 1358–1360 (1991).
Quaia, C., Lefevre, P. & Optican, L.M. Model of the control of saccades by superior colliculus and cerebellum. J. Neurophysiol. 82, 999–1018 (1999).
Klier, E.M., Wang, H. & Crawford, J.D. The superior colliculus encodes gaze commands in retinal coordinates. Nat. Neurosci. 4, 627–632 (2001).
Keller, E.L. & Edelman, J.A. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J. Neurophysiol. 72, 2754–2770 (1994).
Munoz, D.P., Waitzman, D.M. & Wurtz, R.H. Activity of neurons in monkey superior colliculus during interrupted saccades. J. Neurophysiol. 75, 2562–2580 (1996).
Keller, E.L., Gandhi, N.J. & Weir, P.T. Discharge of superior collicular neurons during saccades made to moving targets. J. Neurophysiol. 76, 3573–3577 (1996).
Stanford, T.R. & Sparks, D.L. Systematic errors for saccades to remembered targets: evidence for a dissociation between saccade metrics and activity in the superior colliculus. Vision Res. 34, 93–106 (1994).
Goffart, L. & Pelisson, D. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. I. Gaze dysmetria. J. Neurophysiol. 79, 1942–1958 (1998).
Goffart, L., Pelisson, D. & Guillaume, A. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. II. Dynamics and eye–head coupling. J. Neurophysiol. 79, 1959–1976 (1998).
Pelisson, D., Goffart, L. & Guillaume, A. Contribution of the rostral fastigial nucleus to the control of orienting gaze shifts in the head-unrestrained cat. J. Neurophysiol. 80, 1180–1196 (1998).
Fakhri, S., Pélisson, D. & Guitton, D. Compensation for perturbations of gaze and role of vestibular signals in gaze control. in Information Processing Underlying Gaze Control. (eds. Delgado-Garcia, J.M., Godaux, E. & Vidal, P.P.) 53–63 (Elsevier, New York, 1994).
Chun, K.S. & Robinson, D.A. A model of quick phase generation in the vestibulo-ocular reflex. Biol. Cybern. 28, 209–221 (1978).
Galiana H.L. A nystagmus strategy to linearize the vestibulo-ocular reflex. IEEE Trans. Biomed. Eng. 38, 532–543 (1991).
Paré, M. & Guitton, D. Brain stem omnipause neurons and the control of combined eye–head gaze saccades in the alert cat. J. Neurophysiol. 79, 3060–3076 (1998).
MacPherson, J.M. & Aldridge, J.W. A quantitative method of computer analysis of spike train data collected from behaving animals. Brain Res. 175, 183–187 (1979).
Richmond, B.J., Optican, L.M., Podell, M. & Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J. Neurophysiol. 57, 132–146 (1987)
Acknowledgements
Funded by the Canadian Institutes of Health Research (CIHR). A.B. held a CIHR studentship. S.M. was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture. We thank W.Y. Choi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bergeron, A., Matsuo, S. & Guitton, D. Superior colliculus encodes distance to target, not saccade amplitude, in multi-step gaze shifts. Nat Neurosci 6, 404–413 (2003). https://doi.org/10.1038/nn1027
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nn1027
This article is cited by
-
Fixational drift is driven by diffusive dynamics in central neural circuitry
Nature Communications (2022)
-
The role of the posterior parietal cortex in saccadic error processing
Brain Structure and Function (2020)
-
A competitive integration model of exogenous and endogenous eye movements
Biological Cybernetics (2010)
-
Activation of superior colliculi in humans during visual exploration
BMC Neuroscience (2007)
-
Our eyes deviate away from a location where a distractor is expected to appear
Experimental Brain Research (2006)