Abstract
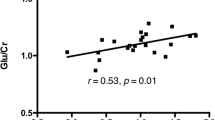
Proton magnetic resonance spectroscopy (1H-MRS) allows the non-invasive measurement of several metabolites, including N-acetyl-aspartate (NAA), an amino acid exclusively synthesized in the mitochondria of neurons, and glutamate, an amino acid involved in excitatory neurotransmission and metabolism. In view of recent postmortem studies in schizophrenia (SZ) revealing mitochondrial abnormalities as well as perturbed expression of the enzymes regulating the glutamate–glutamine cycle, we hypothesized that a disruption in the homeostasis of NAA and glutamate in SZ is present. Fifty subjects with SZ and 48 matched healthy controls (HC) were enrolled in this 1H-MRS study. Voxels were placed in the anterior cingulate cortex (ACC) and hippocampus; NAA/Cr and glutamate + glutamine (Glx)/Cr ratios were obtained. We did not find any significant differences between the groups in metabolite levels in both the ACC and hippocampus. In the hippocampus we found that NAA/Cr and Glx/Cr ratios were significantly correlated in HC (r=0.40, p<0.01 (corrected p=0.048)) but not in SZ (r=−0.06; p=0.71), a difference that was statistically significant (z=2.22, p=0.02). Although no differences in neurometabolites between SZ and HC were apparent, correlations between NAA/Cr and Glx/Cr in healthy subjects in the hippocampus were found, and this correlation was lost in subjects with SZ. To our knowledge, this is the first study to suggest decoupling of these metabolites, a pathophysiological change that may be unique to SZ. However, these results warrant replication and further exploration before definite conclusions can be drawn.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y et al (2005). Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 58: 85–96.
Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská A (2008). Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. J Magn Reson Imaging 27: 489–499.
Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB (1996). Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport 7: 1397–1400.
Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE (2008). Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res 104: 108–120.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007). Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32: 1888–1902.
Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF et al (2001). The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry 49: 39–46.
Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH (2005). Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res 75: 27–34.
Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C et al (2010). 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry 15: 629–636.
Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ et al (2006). N-acetylaspartate as a reservor for glutamate. Med Hypotheses 67: 506–512.
Clay HB, Sillivan S, Konradi C (2011). Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci 29: 311–324.
Dervaux A, Laqueille X (2008). [Smoking and schizophrenia: epidemiological and clinical features]. L’Encéphale 34: 299–305.
Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ (2004). Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res 28: 1849–1860.
Flemenbaum A, Zimmermann RL (1973). Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep 32: 783–792.
Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D et al (2007). Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Pychopharmacol 27: 80–84.
Gallinat J, Schubert F (2007). Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry 40: 64–67.
Garcia M, Huppertz HJ, Ziyeh S, Buechert M, Schumacher M, Mader I (2009). Valproate-induced metabolic changes in patients with epilepsy: assessment with H-MRS. Epilepsia 50: 486–492.
Healy DJ, Meador-Woodruff JH (2000). Ionotropic glutamate receptor modulation preferentially affects NMDA receptor expression in rat hippocampus. Synapse 38: 294–304.
Holm S (1979). A simple sequentially rejective multiple test procedure. Scan J Statist 6: 65–70.
Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X et al (2012). Elevated prefrontal cortex Y-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69: 449–459.
Keltner JR, Wald LL, Christensen JD, Maas LC, Moore CM, Cohen BM et al (1996). A technique for detecting GABA in the human brain with PRESS localization and optimized refocusing spectral editing radiofrequency pulses. Magn Reson Med 36: 458–461.
Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK et al (2011). Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat 2011: 650450.
Kraguljac NV, Reid MA, White D, Jones R, den Hollander J, Lowman D et al (2012). Neurometabolites in schizophrenia and bipolar disorder—a systematic review and metaanalysis. Psychiatry Res; e-pub ahead of print. doi:10.1016/j.psychresns.2012.02.003.
Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995). Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13: 9–19.
López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A (2007). Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology 32: 2087–2097.
Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO et al (2010). Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry 15: 308–318.
Magistretti PJ, Pellerin L (1996). Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci 777: 380–387.
Marenco S, Steele SU, Egan MF, Goldberg TE, Straub RE, Sharrief AZ (2006). Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate measures in the dorsolateral prefrontal cortex. Am J Psychiatry 163: 740–742.
Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE (2011). Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophrenia Bulletin; e-pub ahead of print 11 July 2011. doi:10.1093/schbul/sbr069.
McLoughlin G, Ma D, Tsamg TM, Jones D, Cilia J, Hill M et al (2009). Analysing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res 8: 1943–1952.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA (2007). N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81: 89–131.
Moreno A, Ross BD, Blüml S (2001). Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by (13)C MRS and [1-(13)C]glucose infusion. J Neurochem 77: 347–350.
Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J et al (1994). Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 51: 849–859.
Olbrich HM, Valerius G, Rüsch N, Buchert M, Thiel T, Hennig J et al (2008). Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry 9: 59–63.
Pouwels PJ, Frahm J (1997). Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed 10: 73–78.
Pouwels PJ, Frahm J (1998). Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med 39: 53–60.
Randolph C, Tierney MC, Mohr E, Chase TN (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20: 310–319.
Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS et al (2010). Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry 68: 625–633.
Rosenfeld M, Brenner-Lavie H, Ari SGB, Kavushansky A, Ben-Shachar D (2011). Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry 69: 980–988.
Schubert F, Galliant J, Seifert F, Rinneberg H (2004). Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21: 1762–1771.
Shibuya-Tayoshi S, Tayoshi S, Sumitani S, Ueno S, Harada M, Ohmori T (2008). Lithium effects on brain glutamatergic and GABAergic systems of healthy volunteers as measured by proton magnetic resonance spectroscopy. Prog Neuro-psychopharmacol Biol Psychiatry 32: 249–256.
Steen RG, Hamer RM, Lieberman JA (2005). Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology 30: 1949–1962.
Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH (2008). Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res 103: 71–82.
Szulc A, Galinska B, Tarasow E, Dzienis W, Kubas B, Konarzewska B et al (2005). The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS). Pharmacopsychiatry 38: 214–219.
Tamminga CA, Stan AD, Wagner AD (2010). The hippocampal formation in schizophrenia. Am J Psychiatry 167: 1178–1193.
Théberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RWJ, Rajakumar N et al (2003). Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry 160: 2231–2233.
Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J et al (2002). Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159: 1944–1946.
van Elst LT, Valerius G, Büchert M, Thiel T, Rüsch N, Bubl E et al (2005). Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry 58: 724–730.
Vanhamme L, van den Boogaart A, Van Huffel S (1997). Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43.
Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM et al (2011). Anterior cingulate and cerebellar GABA and Glu correlations measured by 1H J-difference spectroscopy. Magn Reson Imaging 29: 19–24.
Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM et al (2008). Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320: 539–543.
Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U et al (2009). The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66: 478–486.
Weber OM, Trabesinger AH, Duc CO, Meier D, Boesiger P (1997). Detection of hidden metabolites by localized proton magnetic resonance spectroscopy in vivo. Technol Healthcare 5: 471–491.
Wood SJ, Yücel M, Wellard RM, Harrison BJ, Clarke K, Fornito A et al (2007). Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res 94: 328–331.
Acknowledgements
This work was supported by the National Institute of Health (Grant RO1 MH081014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Over the past 2 years, Dr Lahti has received research funds from the National Institute of Health (Grant RO1 MH081014), and an investigator-initiated research grant from Pfizer. Dr Kraguljac has received a research grant from the University of Alabama at Birmingham. Ms Reid, Mr White, and Dr den Hollander declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Kraguljac, N., Reid, M., White, D. et al. Regional Decoupling of N-acetyl-aspartate and Glutamate in Schizophrenia. Neuropsychopharmacol 37, 2635–2642 (2012). https://doi.org/10.1038/npp.2012.126
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.126
Keywords
This article is cited by
-
Higher-order functional brain networks and anterior cingulate glutamate + glutamine (Glx) in antipsychotic-naïve first episode psychosis patients
Translational Psychiatry (2024)
-
Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies
Molecular Psychiatry (2022)
-
Glutamatergic dysfunction is associated with phenotypes of VGF-overexpressing mice
Experimental Brain Research (2022)
-
The relationship between synaptic density marker SV2A, glutamate and N-acetyl aspartate levels in healthy volunteers and schizophrenia: a multimodal PET and magnetic resonance spectroscopy brain imaging study
Translational Psychiatry (2021)
-
Altered anterior cingulate glutamatergic metabolism in depressed adolescents with current suicidal ideation
Translational Psychiatry (2020)