Abstract
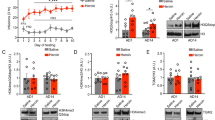
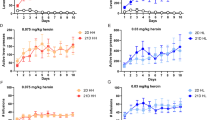
Mu-opioid receptors (MOPRs) are the target of heroin and other prescription opioids, which are currently responsible for massive addiction morbidity in the US. The gene coding for the human MOPR (OPRM1) has an important functional single nucleotide polymorphism (SNP), A118G. The OPRM1 A118G genotype results in substantially increased risk of heroin addiction in humans; however, the neurobiological mechanism for this increased risk is not fully understood. This study examined heroin self-administration (SA) behavior in A112G (G/G) mice, harboring a functionally equivalent SNP in Oprm1 with a similar amino acid substitution, in extended (4 h) SA sessions. Adult male and female G/G mice and ‘wild-type’ litter mates (A/A) were allowed to self-administer heroin (0.25 mg/kg/unit dose, FR1 with a nose poke response) for 4 h/day, for 10 consecutive days. Half of the mice then continued in a heroin dose–response study, while extinction from heroin SA was studied in the other half. In vivo microdialysis was used to measure acute heroin-induced increases of striatal dopamine in the GG vs AA genotypes. Male and female G/G mice responded for heroin significantly more (and thus had greater intake) than A/A mice, in the initial 10 days of heroin SA, and in the subsequent dose–response study. There were no significant differences in extinction of SA between the A/A and G/G mice. Heroin-induced increases in striatal dopamine levels are higher in the GG mice than in the AA mice. Both male and female G/G mice self-administered more heroin than did A/A mice over a 10-day period, possibly because of the greater increases of heroin-induced striatal dopamine in the GG mice. Furthermore, G/G male mice escalated the amount of heroin self-administration across 10 extended-access sessions more than A/A male mice did. These are the first studies to examine the acquisition of heroin SA in this mouse model. These studies may lead to a better understanding of the neurobiological and behavioral mechanisms that underlie greater risk of heroin addiction in carriers of the A118G SNP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J et al (2004). Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry 9: 547–549.
Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L et al (2005). Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology 30: 417–422.
Becker A, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H et al (2000). Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn-Schmiedeberg's Arch Pharmacol 361: 584–589.
Belin D, Everitt BJ (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57: 432–441.
Bond C, LaForge KS, Tian MT, Melia D, Zhang SW, Borg L et al (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci USA 95: 9608–9613.
Boswell MV, Stauble ME, Loyd GE, Langman L, Ramey-Hartung B, Baumgartner RN et al (2013). The role of hydromorphone and OPRM1 in postoperative pain relief with hydrocodone. Pain Physician 16: 227–235.
Charbogne P, Kieffer BL, Befort K (2014). 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76 Pt B: 204–217.
Contet C, Kieffer BL, Befort K (2004). Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol 14: 370–378.
Franklin KBJ, Paxinos G (1997) The Mouse Brain in Stereotaxic Coordinates. Academic Press: New York.
Haerian BS, Haerian MS (2013). OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics 14: 813–824.
Hendershot CS, Claus ED, Ramchandani VA (2014). Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol 2014: 20.
Huang MC, Schwandt ML, Ramchandani VA, George DT, Heilig M (2012). Impact of multiple types of childhood trauma exposure on risk of psychiatric comorbidity among alcoholic inpatients. Alcohol Clin Exp Res 36: 1099–1107.
Johnson SW, North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483–488.
Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA (2005). Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev 57: 1–26.
Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ (2007). The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem 103: 77–87.
LaForge KS, Shick V, Spangler R, Proudnikov D, Yuferov V, Lysov Y et al (2000). Detection of single nucleotide polymorphisms of the human mu opioid receptor gene by hybridization or single nucleotide extension on custom oligonucleotide gelpad microchips: potential in studies of addiction. American J Med Genet 96: 604–615.
Lutz PE, Kieffer BL (2013). The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol 23: 473–479.
Mague SD, Blendy JA (2010). OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108: 172–182.
Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA (2009). Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci USA 106: 10847–10852.
Maisonneuve IM, Kreek MJ et al (1994). Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther 268: 916–921.
Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I et al (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383: 819–823.
Porrino LJ, Smith HR, Nader MA, Beveridge TJ (2007). The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry 31: 1593–1600.
Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H et al (2011). A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry 16: 809–817 PMCID: PMC2925052.
Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF et al (2000). mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther 293: 1002–1008.
Song Z, Du B, Wang K, Shi X. (2013). Effects of OPRM1 A118G polymorphism on epidural analgesia with fentanyl during labor: a meta-analysis. Genet Test Mol Biomarkers 17: 743–749.
Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS et al (2001). Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology 25: 41–54.
Sora I, Funada M, Uhl GR (1997). The mu-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol 324: R1–R2.
Szeto CY, Tang NL, Lee DT, Stadlin A (2001). Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport 12: 1103–1106.
Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR et al (2012). Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 224: 559–571.
Wang YJ, Huang P, Blendy JA, Liu-Chen LY (2012a). Brain region- and sex-specific alterations in DAMGO-stimulated [(35) S]GTPgammaS binding in mice with Oprm1 G/G. Addict Biol 19: 354–361.
Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY (2012b). Reduced expression of the mu opioid receptor in some, but not all, brain regions in mice with OPRM1 G/G. Neuroscience 205: 178–184.
Weerts EM, McCaul ME, Kuwabara H, Yang X, Xu X, Dannals RF et al (2013). Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol 16: 47–53.
Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A et al (2013). Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl) 231: 1277–1287.
Zhang Y, Schlussman SD, Ho A, Kreek MJ (2001). Effect of acute binge cocaine on levels of extracellular dopamine in the caudate putamen and nucleus accumbens in male (57BL/6J and 129/J mice. Brain Res. 923: 172–177.
Zhang Y, Schlussman SD, Ho A, Kreek MJ (2003). Effect of chronic ‘binge cocaine’ on basal levels and cocaine-induced increases of dopamine in the caudate putamen and nucleus accumbens of C57BL/6J and 129/J mice. Synapse 50: 191–199.
Zhang Y, Svenningsson P, Picetti R, Schlussman SD, Nairn AC, Ho A et al (2006). Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32. J Neurosci 26: 2645–2651.
Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W (2005). Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem 280: 32618–32624.
Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ (2006). Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol 191: 137–145.
Acknowledgements
This work was supported by NIH 1R01DA029147 (YZ) and the Dr Miriam and Sheldon G. Adelson Medical Research Foundation (MJK). The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Picetti, R., Butelman, E. et al. Mouse Model of the OPRM1 (A118G) Polymorphism: Differential Heroin Self-Administration Behavior Compared with Wild-Type Mice. Neuropsychopharmacol 40, 1091–1100 (2015). https://doi.org/10.1038/npp.2014.286
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.286
This article is cited by
-
Effect of prenatal and early post-natal oxycodone exposure on the reinforcing and antinociceptive effects of oxycodone in adult C57BL/6 J mice
Psychopharmacology (2024)
-
Enhanced heroin self-administration and distinct dopamine adaptations in female rats
Neuropsychopharmacology (2021)
-
Sex differences in neural mechanisms mediating reward and addiction
Neuropsychopharmacology (2019)
-
Opioid receptors: drivers to addiction?
Nature Reviews Neuroscience (2018)
-
Oprm1 A112G, a single nucleotide polymorphism, alters expression of stress-responsive genes in multiple brain regions in male and female mice
Psychopharmacology (2018)