Abstract
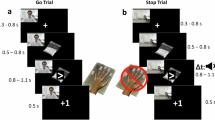
Although reward processing appears altered in addiction, few studies track neurofunctional changes following treatment or relate these to measures of reduced drug use. The current study examined neurofunctional alterations in reward processing in cocaine dependence (CD) pretreatment and posttreatment to determine whether these changes relate to clinically meaningful outcome indicators. Treatment-seeking CD outpatients (N=29) underwent functional magnetic resonance imaging while performing a monetary incentive delay task (MIDT) pretreatment and posttreatment. The MIDT parses anticipatory from outcome phases of reward/loss processing. Abstinence indicators (negative urines, days abstinent from cocaine during follow-up) were collected throughout treatment and up to 1 year later. Healthy control (HC) participants (N=28) were also scanned twice with the MIDT. Relative to pretreatment, at posttreatment CD participants demonstrated increased anticipatory reward activity in the midbrain, thalamus, and precuneus (pFWE<0.05). Increased midbrain activity correlated with cocaine abstinence during the 1-year follow-up. Ventral striatal (VS) activity during loss anticipation correlated negatively with negative urine screens. HC group test–retest results showed decreased ventromedial prefrontal cortex activity during winning outcomes. CD–HC group-by-time differences revealed increased left inferior frontal gyrus activity in the CD group during anticipatory phases at posttreatment. In CD participants, increased posttreatment activity in dopamine-innervated regions suggests lowered thresholds in anticipatory signaling for non-drug rewards. Midbrain and VS responses may represent biomarkers associated with CD abstinence. Abstinence-related neurobiological changes occur in similar regions implicated during active use and may possibly be used to track progress during short- and long-term recovery.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D et al (2010). Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse 64: 397–402.
Balodis IM, Potenza MN (2015). Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry 77: 434–444.
Bari A, Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79.
Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M et al (2007). Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 27: 3998–4003.
Bustamante JC, Barros-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N et al (2013). Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol. 19: 885–894.
Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D et al (2014). Toward empirical identification of a clinically meaningful indicator of treatment outcome: features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug Alcohol Depend 137: 3–19.
Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA et al (2016). A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug Alcohol Depend pii: S0376-8716(16)00008-9. doi:10.1016/j.drugalcdep.2015.12.036 (e-pub ahead of print).
Choi JK, Chen YI, Hamel E, Jenkins BG (2006). Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage 30: 700–712.
Corbetta M, Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215.
Everitt BJ, Robbins TW (2013). From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37: 1946–1954.
Garavan H, Brennan KL, Hester R, Whelan R (2013). The neurobiology of successful abstinence. Curr Opin Neurobiol 23: 668–674.
Goldstein RZ, Tomasi D, Alia-Klein N, Honorio Carrillo J, Maloney T, Woicik PA et al (2009). Dopaminergic response to drug words in cocaine addiction. J Neurosci 29: 6001–6006.
Hagele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F et al (2015). Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 232: 331–341.
Hanlon CA, Beveridge TJ, Porrino LJ (2013). Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci Biobehav Rev 37: 2037–2046.
Heyman GM (2013). Quitting drugs: quantitative and qualitative features. Annu Rev Clin Psychol 9: 29–59.
Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD et al (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry 70: 553–560.
Knutson B, Adams CM, Fong GW, Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159.
Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18: 263–272.
Konova AB, Moeller SJ, Goldstein RZ (2013). Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci Biobehav Rev 37: 2806–2817.
Kuhn S, Gallinat J (2011). Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33: 1318–1326.
Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J et al (2012). Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict Biol 17: 1013–1025.
Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN et al (2013). Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol Psychiatry 74: 529–537.
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618.
Prendergast M, Podus D, Finney J, Greenwell L, Roll J (2006). Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 101: 1546–1560.
Raj D, Anderson AW, Gore JC (2001). Respiratory effects in human functional magnetic resonance imaging due to bulk susceptibility changes. Phys Med Biol 46: 3331–3340.
Sarter M, Gehring WJ, Kozak R (2006). More attention must be paid: the neurobiology of attentional effort. Brain Res Rev 51: 145–160.
Saunders BT, Yager LM, Robinson TE (2013). Cue-evoked cocaine "craving": role of dopamine in the accumbens core. J Neurosci 33: 13989–14000.
Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599.
Vocci FJ (2007). Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction 102: 1888–1889.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64: 1575–1579.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR et al (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M et al (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27: 12700–12706.
Wager TD, Keller MC, Lacey SC, Jonides J (2005). Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26: 99–113.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A et al (2006). Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31: 2716–2727.
Woo CW, Krishnan A, Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91: 412–419.
Acknowledgements
This study was supported by the National Institute on Drug Abuse grants R01 DA019078, P50 DA09241, P20 DA027844, R01 DA035058, R01 DA020908, and K12 DA00167.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balodis, I., Kober, H., Worhunsky, P. et al. Neurofunctional Reward Processing Changes in Cocaine Dependence During Recovery. Neuropsychopharmacol 41, 2112–2121 (2016). https://doi.org/10.1038/npp.2016.11
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.11
This article is cited by
-
Change in brain activation after transcranial pulsed electromagnetic fields in treatment-resistant depression
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Residual deficits in functional brain activity after chronic cocaine self-administration in rhesus monkeys
Neuropsychopharmacology (2023)
-
Prior cocaine self-administration impairs attention signals in anterior cingulate cortex
Neuropsychopharmacology (2020)
-
Effects of familial risk and stimulant drug use on the anticipation of monetary reward: an fMRI study
Translational Psychiatry (2019)
-
Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research
Neuropsychopharmacology (2019)