Abstract
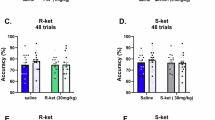
Nr4a nuclear receptors contribute to long-term memory formation and are required for long-term memory enhancement by a class of broad-acting drugs known as histone deacetylase (HDAC) inhibitors. Understanding the molecular mechanisms that regulate these genes and identifying ways to increase their activity may provide novel therapeutic approaches for ameliorating cognitive dysfunction. In the present study, we find that Nr4a gene expression after learning requires the cAMP-response element binding (CREB) interaction domain of the histone acetyltransferase CREB-binding protein (CBP). These gene expression deficits emerge at a time after learning marked by promoter histone acetylation in wild-type mice. Further, mutation of the CREB–CBP interaction domain reduces Nr4a promoter acetylation after learning. As memory enhancement by HDAC inhibitors requires CREB–CBP interaction and Nr4a gene function, these data support the notion that the balance of histone acetylation at the Nr4a promoters is critical for memory formation. NR4A ligands have recently been described, but the effect of these drugs on synaptic plasticity or memory has not been investigated. We find that the ‘C-DIM’ NR4A ligands, para-phenyl substituted di-indolylmethane compounds, enhance long-term contextual fear memory and increase the duration of long-term potentiation (LTP), a form of hippocampal synaptic plasticity. LTP enhancement by these drugs is eliminated in mice expressing a dominant negative form of NR4A and attenuated in mice with mutation of the CREB–CBP interaction domain. These data define the molecular connection between histone acetylation and Nr4a gene expression after learning. In addition, they suggest that NR4A-activating C-DIM compounds may serve as a potent and selective means to enhance memory and synaptic plasticity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER et al (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947–959.
Bridi MS, Abel T (2013a) Histone modifications in the nervous system and neuropsychiatric disorders. In: Sweatt JD, Meaney MJ, Nestler E, Akbarian S (eds). Epigenetic Regulation in the Nervous System. Elsevier: Waltham, pp 35–67.
Bridi MS, Abel T (2013b). The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiol Learn Mem 105: 151–158.
Chintharlapalli S, Smith R, Samudio I, Zhang W, Safe S (2004). 1,1-Bis(3’-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor gamma-mediated growth inhibition, transactivation, and differentiation markers in colon cancer cells. Cancer Res 64: 5994–6001.
Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S et al (2007). Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res 67: 674–683.
Colón-Cesario WI, Martínez-Montemayor MM, Morales S, Félix J, Cruz J, Adorno M et al (2006). Knockdown of Nurr1 in the rat hippocampus: implications to spatial discrimination learning and memory. Learn Mem 13: 734–744.
De Miranda BR, Miller JA, Hansen RJ, Lunghofer PJ, Safe S, Gustafson DL et al (2013). Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson’s disease. J Pharmacol Exp Ther 345: 125–138.
De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, Tjalkens RB (2015). Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci 2: 360–373.
Dubois C, Hengerer B, Mattes H (2006). Identification of a potent agonist of the orphan nuclear receptor Nurr1. ChemMedChem 1: 955–958.
Fanselow MS (1980). Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15: 177–182.
Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao J et al (2009). HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60.
Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA (2011). HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18: 71–79.
Hawk JD, Bookout AL, Poplawski SG, Bridi MS, Rao AJ, Sulewski ME et al (2012). NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. J Clin Invest 122: 3593–3602.
Hawk JD, Florian C, Abel T (2011). Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem 18: 367–370.
Hebbes TR, Thorne AW (1988). A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 7: 1395–1402.
Hintermann S, Chiesi M, von Krosigk U, Mathé D, Felber R, Hengerer B (2007). Identification of a series of highly potent activators of the Nurr1 signaling pathway. Bioorg Med Chem Lett 17: 193–196.
Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM et al (2004). Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119: 1041–1054.
Inamoto T, Papineni S, Chintharlapalli S, Cho S-D, Safe S, Kamat AM (2008). 1,1-Bis(3’-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther 7: 3825–3833.
Johnson MM, Michelhaugh SK, Bouhamdan M, Schmidt CJ, Bannon MJ (2011). The transcription factor NURR1 exerts concentration-dependent effects on target genes mediating distinct biological processes. Front Neurosci 5: 135.
Kasper LH, Boussouar F, Ney PA, Jackson CW, Rehg J, van Deursen JM et al (2002). A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature 419: 738–743.
Korzus E, Rosenfeld MG, Mayford M (2004). CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961–972.
Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA et al (2002). Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, Protein Kinase A, and MAPK pathways. Mol Endocrinol 16: 1638–1651.
Lattal KM, Barrett RM, Wood MA (2007). Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci 121: 1125–1131.
Lee S-O, Li X, Hedrick E, Jin U-H, Tjalkens RB, Backos DS et al (2014). Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol Endocrinol 28: 1729–1739.
Lee SO, Chintharlapalli S, Liu S, Papineni S, Cho SD, Yoon K et al (2009). p21 expression is induced by activation of nuclear nerve growth factor-induced Balpha (Nur77) in pancreatic cancer cells. Mol Cancer Res 7: 1169–1178.
Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S (2008). 1,1-Bis(3’-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-jun N-terminal kinase. Carcinogenesis 29: 1139–1147.
Lemberger T, Parkitna JR, Chai M, Schütz G, Engblom D (2008). CREB has a context-dependent role in activity-regulated transcription and maintains neuronal cholesterol homeostasis. FASEB J 22: 2872–2879.
Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004). Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559.
Li X, Lee S-O, Safe S (2012). Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3’-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochem Pharmacol 83: 1445–1455.
Lopez-Atalaya JP, Barco A (2014). Can changes in histone acetylation contribute to memory formation? Trends Genet 30: 529–539.
Malvaez M, McQuown SC, Rogge G a, Astarabadi M, Jacques V, Carreiro S et al (2013). HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci USA 110: 2647–2652.
Maren S, Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neuroservice 5: 844–852.
Maxwell MA, Muscat GEO (2006). The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 4: e002.
Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER (1996). Control of memory formation through regulated expression of a CaMKII transgene. Science (80-) 277: 1678–1683.
McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF et al (2012). Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19: 588–592.
Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M et al (1999). Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet 8: 387–396.
Ordentlich P, Yan Y, Zhou S, Heyman RA (2003). Identification of the antineoplastic agent 6-mercaptopurine as an activator of the orphan nuclear hormone receptor Nurr1. J Biol Chem 278: 24791–24799.
Park AJ, Havekes R, Choi JHK, Luczak V, Nie T, Huang T et al (2014). A presynaptic role for PKA in synaptic tagging and memory. Neurobiol Learn Mem 114: 101–112.
Pearen MA, Muscat GEO (2010). Minireview: nuclear hormone receptor 4A signaling: Implications for metabolic disease. Mol Endocrinol 24: 1891–1903.
Peixoto L, Abel T (2013). The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38: 62–76.
Poplawski SG, Schoch H, Wimmer ME, Hawk JD, Walsh JL, Giese KP et al (2014). Object-location training elicits an overlapping but temporally distinct transcriptional profile from contextual fear conditioning. Neurobiol Learn Mem 116: 90–95.
Robert NM, Martin LJ, Tremblay JJ (2006). The orphan nuclear receptor NR4A1 regulates insulin-like 3 gene transcription in Leydig cells. Biol Reprod 74: 322–330.
Rogge G a., Singh H, Dang R, Wood MA (2013). HDAC3 is a negative regulator of cocaine-context-associated memory formation. J Neurosci 33: 6623–6632.
Rojas P, Joodmardi E, Hong Y, Perlmann T, Ogren SO (2007). Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry 12: 756–766.
Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA (2009). Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA 106: 9447–9452.
Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA et al (2007). Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140.
Wansa KDSA, Harris JM, Yan G, Ordentlich P, Muscat GEO (2003). The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem 278: 24776–24790.
Wood MA, Attner MA, Oliveira AMM, Brindle PK, Abel T (2006). A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 13: 609–617.
Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AMM, Lombardi TL et al (2005). Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119.
Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D et al (2008). Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol 4: 548–556.
Acknowledgements
The manuscript was written by MSB and JDH, with suggestions from TA and SS. MSB, JDH, SC, and TA conceived and designed the experiments. MSB conducted electrophysiology experiments. JDH conducted gene expression and ChIP experiments. SC conducted behavioral experiments. TA directed the studies. Thanks to Dr Michelle Bridi for providing comments and feedback on the manuscript, and thanks to Dr W. Timothy O’Brien and the University of Pennsylvania Neurobehavior Testing Core for assistance with fear conditioning experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bridi, M., Hawk, J., Chatterjee, S. et al. Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Neuropsychopharmacol 42, 1243–1253 (2017). https://doi.org/10.1038/npp.2016.253
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.253
This article is cited by
-
HDAC3 as an Emerging Therapeutic Target for Alzheimer's Disease and other Neurological Disorders
Molecular Neurobiology (2025)
-
Mapping the spatial transcriptomic signature of the hippocampus during memory consolidation
Nature Communications (2023)
-
Cocaine regulation of Nr4a1 chromatin bivalency and mRNA in male and female mice
Scientific Reports (2022)
-
Cocaine induces paradigm-specific changes to the transcriptome within the ventral tegmental area
Neuropsychopharmacology (2021)
-
I2 imidazoline receptor modulation protects aged SAMP8 mice against cognitive decline by suppressing the calcineurin pathway
GeroScience (2021)