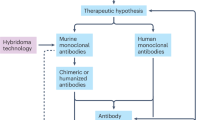
Key Points
-
Molecular engineering has enabled the fine-tuning of monoclonal antibody (mAb) function to enhance their effects and to minimize immunogenicity and side effects. In this article we take a closer look at the safety and side effects of currently available mAbs.
-
Acute infusion reactions can be caused by a range of mechanisms including anaphylaxis, anaphylactoid reactions, serum sickness, tumour lysis syndrome and cytokine release syndrome.
-
mAbs against tumour necrosis factor-α (TNFα) have been associated with reactivation of latent tuberculosis, as well as with other serious infections and malignancies.
-
Progressive multifocal leukoencephalopathy is a rare but serious complication of natalizumab (Tysabri; Biogen Idec, Elan), rituximab (Rituxan/MabThera; Genentech, Biogen Idec) and efalizumab (Raptiva; Genentech).
-
Treatment with abciximab (ReoPro; Centocor Ortho Biotech, Eli Lilly), an antiplatelet glycoprotein IIb/IIIa chimeric Fab antibody fragment, can cause thrombocytopaenia; although it can also be caused by various other mAbs due to immune thrombocytopaenia.
-
mAbs directed against TNFα can cause a lupus-like syndrome; alemtuzumab (Campath; Genzyme) can mediate thyroid disease through autoimmunity; and mAbs directed against cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) can initiate autoimmune colitis.
-
mAbs against human epidermal growth factor receptor commonly cause skin rashes, while trastuzumab (Herceptin; Genentech), an ERBB2-specific mAb, can cause cardiotoxicity.
-
The dramatic cytokine storm seen after infusion of TGN1412 (a CD28 superagonist) has resulted in the recommendation of a range of measures to improve the safety of first-in-human clinical testing with mAbs.
Abstract
Monoclonal antibodies (mAbs) are now established as targeted therapies for malignancies, transplant rejection, autoimmune and infectious diseases, as well as a range of new indications. However, administration of mAbs carries the risk of immune reactions such as acute anaphylaxis, serum sickness and the generation of antibodies. In addition, there are numerous adverse effects of mAbs that are related to their specific targets, including infections and cancer, autoimmune disease, and organ-specific adverse events such as cardiotoxicity. In March 2006, a life-threatening cytokine release syndrome occurred during a first-in-human study with TGN1412 (a CD28-specific superagonist mAb), resulting in a range of recommendations to improve the safety of initial human clinical studies with mAbs. Here, we review some of the adverse effects encountered with mAb therapies, and discuss advances in preclinical testing and antibody technology aimed at minimizing the risk of these events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975). The original manuscript describing the breakthrough of hybridoma technology and the production of mAbs.
Strebhardt, K. & Ullrich, A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nature Rev. Cancer 8, 473–480 (2008).
Dubel, S. (ed.) Handbook of Therapeutic Antibodies. Volume I: Technologies, Volume II: Emerging Developments, Volume III: Approved Therapeutics (Wiley, Weinhem, 2007). A comprehensive three volume multiple-author text on therapeutic antibodies.
Lonberg, N. Human antibodies from transgenic animals. Nature Biotech. 23, 1117–1125 (2005).
Reichert, J. M., Rosensweig, C. J., Faden, L. B. & Dewitz, M. C. Monoclonal antibody successes in the clinic. Nature Biotech. 23, 1073–1078 (2005).
Waldmann, T. A. Immunotherapy: past, present and future. Nature Med. 9, 269–277 (2003).
Reichert, J. M. & Dewitz, M. C. Anti-infective monoclonal antibodies: perils and promise of development. Nature Rev. Drug Discov. 5, 191–195 (2006).
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nature Rev. Drug Discov. 7, 21–39 (2008).
Waldmann, H. & Hale, G. CAMPATH: from concept to clinic. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1707–1711 (2005).
Nissim, A. & Chernajovsky, Y. Historical development of monoclonal antibody therapeutics. Handb. Exp. Pharmacol. 181, 3–18 (2008).
Presta, L. G. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 20, 460–470 (2008).
Hale, G. Therapeutic antibodies-delivering the promise? Adv. Drug Deliv. Rev 58, 633–639 (2006).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Giezen, T. J. et al. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA 300, 1887–1896 (2008). An important review of regulatory actions regarding the safety of biologics.
Zink, A. et al. European biologicals registers: methodology, selected results and perspectives. Ann. Rheum. Dis. 68, 1240–1246 (2009).
Lutterotti, A. & Martin, R. Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol. 7, 538–547 (2008).
Yeung, Y. A. et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182, 7663–7671 (2009).
Chang, T. W. Developing antibodies for targeting immunoglobulin and membrane-bound immunoglobulin E. Allergy Asthma Proc. 27, S7–S14 (2006).
Hassan, M. S., bedi-Valugerdi, M., Lefranc, G., Hammarstrom, L. & Smith, C. I. Biological half-life of normal and truncated human IgG3 in SCID mice. Eur. J. Immunol. 21, 1319–1322 (1991).
Jefferis, R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 30, 356–362 (2009).
Holland, M. et al. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener's granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin. Exp. Immunol. 138, 183–192 (2004).
van der Neut Kolfschoten, M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007).
Tabrizi, M. A. & Roskos, L. K. Preclinical and clinical safety of monoclonal antibodies. Drug Discov. Today 12, 540–547 (2007).
Cavagnaro, J. A. (ed.) Preclinical Safety Evaluation of Biopharmaceuticals: A Science Based Approach to Facilitating Clinical Trials (Wiley, London, 2008). A recent book on preclinical safety testing of biopharmaceuticals.
Longstaff, C., Whitton, C. M., Stebbings, R. & Gray, E. How do we assure the quality of biological medicines? Drug Discov. Today 14, 50–55 (2009).
Loisel, S. et al. Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment. Crit. Rev. Oncol. Hematol. 62, 34–42 (2007).
Chapman, K., Pullen, N., Graham, M. & Ragan, I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nature Rev. Drug Discov. 6, 120–126 (2007).
Presta, L. G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Deliv. Rev. 58, 640–656 (2006).
Chung, C. H. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 13, 725–732 (2008).
Klastersky, J. Adverse effects of the humanized antibodies used as cancer therapeutics. Curr. Opin. Oncol. 18, 316–320 (2006).
Kang, S. P. & Saif, M. W. Infusion-related and hypersensitivity reactions of monoclonal antibodies used to treat colorectal cancer — identification, prevention, and management. J. Support. Oncol. 5, 451–457 (2007).
Lenz, H. J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12, 601–609 (2007).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008).
Poole, J. A., Matangkasombut, P. & Rosenwasser, L. J. Targeting the IgE molecule in allergic and asthmatic diseases: review of the IgE molecule and clinical efficacy. J. Allergy Clin. Immunol. 115, S376–S385 (2005).
Gould, H. J. & Sutton, B. J. IgE in allergy and asthma today. Nature Rev. Immunol. 8, 205–217 (2008).
Cox, L. et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J. Allergy Clin. Immunol. 120, 1373–1377 (2007).
Corren, J. et al. Safety and tolerability of omalizumab. Clin. Exp. Allergy 39, 788–797 (2009).
Cox, L. S. How safe are the biologicals in treating asthma and rhinitis? Allergy Asthma Clin. Immunol. 5, 4 (2009).
Limb, S. L., Starke, P. R., Lee, C. E. & Chowdhury, B. A. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J. Allergy Clin. Immunol. 120, 1378–1381 (2007).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Naure Rev. Cancer 1, 118–129 (2001).
Loertscher, R. The utility of monoclonal antibody therapy in renal transplantation. Transplant. Proc. 34, 797–800 (2002).
Gaston, R. S. et al. OKT3 first-dose reaction: association with T cell subsets and cytokine release. Kidney Int. 39, 141–148 (1991).
Kuus-Reichel, K. et al. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1, 365–372 (1994).
Mascelli, M. A. et al. Molecular, biologic, and pharmacokinetic properties of monoclonal antibodies: impact of these parameters on early clinical development. J. Clin. Pharmacol. 47, 553–565 (2007).
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Azinovic, I. et al. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol. Immunother. 55, 1451–1458 (2006).
Clark, M. Antibody humanization: a case of the 'Emperor's new clothes'? Immunol. Today 21, 397–402 (2000).
Ransohoff, R. M. Natalizumab for multiple sclerosis. N. Engl. J. Med. 356, 2622–2629 (2007).
Cohen, B. A., Oger, J., Gagnon, A. & Giovannoni, G. The implications of immunogenicity for protein-based multiple sclerosis therapies. J. Neurol. Sci. 275, 7–17 (2008).
Schellekens, H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol. Dial. Transplant. 20 (Suppl. 6), vi3–vi9 (2005).
Todd, D. J. & Helfgott, S. M. Serum sickness following treatment with rituximab. J. Rheumatol. 34, 430–433 (2007).
Schellekens, H., Crommein, D. & Jiskoot, W. in Handbook of Therapeutic Antibodies Vol. 1 Ch. 11 (ed. Dubel, S.) (Wiley, Weinheim, 2007).
Shankar, G., Shores, E., Wagner, C. & Mire-Sluis, A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol. 24, 274–280 (2006).
Aarden, L., Ruuls, S. R. & Wolbink, G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr. Opin. Immunol. 20, 431–435 (2008).
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. Doc. Ref. EMEA/CHMP/BMWP/14327/2006. EMA website [online], (2007).
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Concept paper on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. Doc. Ref. EMEA/CHMP/BMWP/114720/2009. EMA website [online], (2009). Recent EMA guidelines on immunogenicity testing of mAbs.
Coiffier, B., Altman, A., Pui, C. H., Younes, A. & Cairo, M. S. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J. Clin. Oncol. 26, 2767–2778 (2008).
Tosi, P. et al. Consensus conference on the management of tumor lysis syndrome. Haematologica 93, 1877–1185 (2008).
Otrock, Z. K., Hatoum, H. A. & Salem, Z. M. Acute tumor lysis syndrome after rituximab administration in Burkitt's lymphoma. Intern. Emerg. Med. 3, 161–163 (2008).
Feusner, J. H., Ritchey, A. K., Cohn, S. L. & Billett, A. L. Management of tumor lysis syndrome: need for evidence-based guidelines. J. Clin. Oncol. 26, 5657–5658 (2008).
Taylor, P. C. & Feldmann, M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nature Rev. Rheumatol. 5, 578–582 (2009).
Feldmann, M. & Maini, S. R. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol. Rev. 223, 7–19 (2008).
Moss, M. L., Sklair-Tavron, L. & Nudelman, R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nature Clin. Pract. Rheumatol. 4, 300–309 (2008).
Keane, J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology 44, 714–720 (2005).
Askling, J. et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 52, 1986–1992 (2005).
Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 (2006).
Schneeweiss, S. et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 56, 1754–1764 (2007).
Theis, V. S. & Rhodes, J. M. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 27, 19–30 (2008).
Colombel, J. F. et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology 126, 19–31 (2004).
British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-α treatment. Thorax 60, 800–805 (2005).
Major, E. O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 61, 35–47 (2010).
Carson, K. R. et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) project. Lancet Oncol. 10, 816–824 (2009).
Lopez-Diego, R. S. & Weiner, H. L. Novel therapeutic strategies for multiple sclerosis — a multifaceted adversary. Nature Rev. Drug Discov. 7, 909–925 (2008).
Sadiq, S. A., Puccio, L. M. & Brydon, E. W. JCV detection in multiple sclerosis patients treated with natalizumab. J. Neurol. 7 Jan 2010 (doi:10.1007/s00415-009-5444-4).
Major, E. O. Reemergence of PML in natalizumab-treated patients — new cases, same concerns. N. Engl. J. Med. 361, 1041–1043 (2009).
Kleinschmidt-DeMasters, B. K. & Tyler, K. L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 353, 369–374 (2005).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005).
Van Assche, G. et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353, 362–368 (2005). References 77–79 are the original descriptions of cases of PML with natalizumab.
Wenning, W. et al. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N. Engl. J. Med. 361, 1075–1080 (2009).
Linda, H. et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N. Engl. J. Med. 361, 1081–1087 (2009). References 80 and 81 are recent descriptions of cases of PML with natalizumab.
Yousry, T. A. et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354, 924–933 (2006).
Kappos, L. et al. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol. 6, 431–441 (2007).
Clifford, D. B. Natalizumab and PML: a risky business? Gut 57, 1347–1349 (2008).
Landry, M. L., Eid, T., Bannykh, S. & Major, E. False negative PCR despite high levels of JC virus DNA in spinal fluid: implications for diagnostic testing. J. Clin. Virol. 43, 247–249 (2008).
Chen, Y. et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 1067–1074 (2009).Documentation that reactivation of JCV occurs commonly with natalizumab therapy in multiple sclerosis.
Delbue, S., Tremolada, S. & Ferrante, P. Application of molecular tools for the diagnosis of central nervous system infections. Neurol. Sci. 29 (Suppl. 2), 283–285 (2008).
Molloy, E. S. & Calabrese, L. H. Therapy: targeted but not trouble-free: efalizumab and PML. Nature Rev. Rheumatol. 5, 418–419 (2009).
Bonig, H., Wundes, A., Chang, K. H., Lucas, S. & Papayannopoulou, T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood 111, 3439–3441 (2008).
Zohren, F. et al. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood 111, 3893–3895 (2008).
Aksoy, S. et al. Rituximab-related viral infections in lymphoma patients. Leuk. Lymphoma 48, 1307–1312 (2007).
Carson, K. R. et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840 (2009).
Aster, R. H. & Bougie, D. W. Drug-induced immune thrombocytopenia. N. Engl. J. Med. 357, 580–587 (2007).
Topol, E. J., Byzova, T. V. & Plow, E. F. Platelet GPIIb-IIIa blockers. Lancet 353, 227–231 (1999).
Tcheng, J. E. et al. Abciximab readministration: results of the ReoPro Readministration Registry. Circulation 104, 870–875 (2001).
Topol, E. J. et al. Multi-year follow-up of abciximab therapy in three randomized, placebo-controlled trials of percutaneous coronary revascularization. Am. J. Med. 113, 1–6 (2002).
Tamhane, U. U. & Gurm, H. S. The chimeric monoclonal antibody abciximab: a systematic review of its safety in contemporary practice. Expert Opin. Drug Saf. 7, 809–819 (2008).
Curtis, B. R., Divgi, A., Garritty, M. & Aster, R. H. Delayed thrombocytopenia after treatment with abciximab: a distinct clinical entity associated with the immune response to the drug. J. Thromb. Haemost. 2, 985–992 (2004).
Curtis, B. R., Swyers, J., Divgi, A., McFarland, J. G. & Aster, R. H. Thrombocytopenia after second exposure to abciximab is caused by antibodies that recognize abciximab-coated platelets. Blood 99, 2054–2059 (2002).
McCorry, R. B. & Johnston, P. Fatal delayed thrombocytopenia following abciximab therapy. J. Invasive Cardiol. 18, E173–E174 (2006).
Mukherjee, D. & Roffi, M. Glycoprotein IIb/IIIa receptor inhibitors in 2008: do they still have a role? J. Interv. Cardiol. 21, 118–121 (2008).
Cox, A. L. et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur. J. Immunol. 35, 3332–3342 (2005).
Lorenzi, A. R. et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis Rheum. 58, 370–375 (2008).
Chakrabarti, S. et al. T-cell depletion with Campath-1H “in the bag” for matched related allogeneic peripheral blood stem cell transplantation is associated with reduced graft-versus-host disease, rapid immune constitution and improved survival. Br. J. Haematol. 121, 109–118 (2003).
Hale, G. et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant. 26, 69–76 (2000).
Lin, T. S. Novel agents in chronic lymphocytic leukemia: efficacy and tolerability of new therapies. Clin. Lymphoma Myeloma. 8 (Suppl. 4), 137–143 (2008).
Watson, C. J. et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation — efficacy and safety at five years. Am. J. Transplant. 5, 1347–1353 (2005).
Coles, A. J. et al. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Hauser, S. L. Multiple lessons for multiple sclerosis. N. Engl. J. Med. 359, 1838–1841 (2008).
Haider, I. & Cahill, M. Fatal thrombocytopaenia temporally related to the administration of alemtuzumab (MabCampath) for refractory CLL despite early discontinuation of therapy. Hematology 9, 409–411 (2004).
Gibbs, S. D., Westerman, D. A., McCormack, C., Seymour, J. F. & Miles, P. H. Severe and prolonged myeloid haematopoietic toxicity with myelodysplastic features following alemtuzumab therapy in patients with peripheral T-cell lymphoproliferative disorders. Br. J. Haematol. 130, 87–91 (2005).
Patel, V. L., Schwartz, J. & Bussel, J. B. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br. J. Haematol. 141, 545–548 (2008).
Koyama, I. et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation 77, 460–462 (2004).
Kawai, T., Andrews, D., Colvin, R. B., Sachs, D. H. & Cosimi, A. B. Letters to the Editor: Thromboembolic complications after treatment with monoclonal antibody against CD49 ligand. Nature Med. 6, 114 (2000).
Kirk, A. D. & Harlan, D. M. Letters to the Editor: Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nature Med. 6, 114 (2000).
Mirabet, M., Barrabes, J. A., Quiroga, A. & Garcia-Dorado, D. Platelet pro-aggregatory effects of CD40L monoclonal antibody. Mol. Immunol. 45, 937–944 (2008).
Langer, F. et al. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb. Haemost. 93, 1137–1146 (2005).
Scappaticci, F. A. et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl Cancer Inst. 99, 1232–1239 (2007).
Nalluri, S. R., Chu, D., Keresztes, R., Zhu, X. & Wu, S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300, 2277–2285 (2008).
Mongey, A. B. & Hess, E. V. Drug insight: autoimmune effects of medications — what's new? Nature Clin. Pract. Rheumatol. 4, 136–144 (2008).
Ramos-Casals, M. et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine 86, 242–251 (2007).
Haraoui, B. & Keystone, E. Musculoskeletal manifestations and autoimmune diseases related to new biologic agents. Curr. Opin. Rheumatol. 18, 96–100 (2006).
Coles, A. J. et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 354, 1691–1695 (1999).
Fong, L. & Small, E. J. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J. Clin. Oncol. 26, 5275–5283 (2008).
Maker, A. V., Attia, P. & Rosenberg, S. A. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 175, 7746–7754 (2005).
Peggs, K. S., Quezada, S. A., Korman, A. J. & Allison, J. P. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 18, 206–213 (2006).
Weber, J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist 12, 864–872 (2007).
Kaufman, H. L. & Wolchok, J. D. Is tumor immunity the same thing as autoimmunity? Implications for cancer immunotherapy. J. Clin. Oncol. 24, 2230–2232 (2006).
Askling, J. & Bongartz, T. Malignancy and biologic therapy in rheumatoid arthritis. Curr. Opin. Rheumatol. 20, 334–339 (2008).
Scott, D. L. & Kingsley, G. H. Tumor necrosis factor inhibitors for rheumatoid arthritis. N. Engl. J. Med. 355, 704–712 (2006).
Dixon, W. & Silman, A. Is there an association between anti-TNF monoclonal antibody therapy in rheumatoid arthritis and risk of malignancy and serious infection? Commentary on the meta-analysis by Bongartz. et al. Arthritis Res. Ther. 8, 111 (2006).
Askling, J. et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann. Rheum. Dis. 64, 1421–1426 (2005).
Setoguchi, S. et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 54, 2757–2764 (2006).
Biancone, L., Calabrese, E., Petruzziello, C. & Pallone, F. Treatment with biologic therapies and the risk of cancer in patients with IBD. Nature Clin. Pract. Gastroenterol. Hepatol. 4, 78–91 (2007).
Rennard, S. I. et al. The safety and efficacy of infliximab in moderate-to-severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 175, 926–934 (2007).
Rosh, J. R., Gross, T., Mamula, P., Griffiths, A. & Hyams, J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn's disease: a cautionary tale? Inflamm. Bowel Dis. 13, 1024–1030 (2007).
Krueger, G. G. et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N. Engl. J. Med. 356, 580–592 (2007).
Sandborn, W. J. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology 135, 1442–1447 (2008).
Segal, B. M. et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing–remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008).
Weiss, J. M., Subleski, J. J., Wigginton, J. M. & Wiltrout, R. H. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert. Opin. Biol. Ther. 7, 1705–1721 (2007).
Langowski, J. L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Knox, S. J. et al. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin. Cancer Res. 2, 457–470 (1996).
Witzig, T. E. et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer 109, 1804–1810 (2007).
Jean, G. W. & Shah, S. R. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy 28, 742–754 (2008).
Perez-Soler, R. & Saltz, L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J. Clin. Oncol. 23, 5235–5246 (2005).
Bianchini, D., Jayanth, A., Chua, Y. J. & Cunningham, D. Epidermal growth factor receptor inhibitor-related skin toxicity: mechanisms, treatment, and its potential role as a predictive marker. Clin. Colorectal Cancer 7, 33–43 (2008).
Saif, M. W., Longo, W. L. & Israel, G. Correlation between rash and a positive drug response associated with bevacizumab in a patient with advanced colorectal cancer. Clin. Colorectal Cancer 7, 144–148 (2008).
Bauer, K. A., Hammerman, S., Rapoport, B. & Lacouture, M. E. Completeness in the reporting of dermatologic adverse drug reactions associated with monoclonal antibody epidermal growth factor receptor inhibitors in phase II and III colorectal cancer clinical trials. Clin. Colorectal Cancer 7, 309–314 (2008).
Bernier, J. et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann. Oncol. 19, 142–149 (2008).
Scope, A. et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J. Clin. Oncol. 25, 5390–5396 (2007).
Hudis, C. A. Trastuzumab — mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Force, T., Krause, D. S. & Van Etten, R. A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Rev. Cancer 7, 332–344 (2007).
Guglin, M., Cutro, R. & Mishkin, J. D. Trastuzumab-induced cardiomyopathy. J. Card. Fail. 14, 437–444 (2008).
Klein, P. M. & Dybdal, N. Trastuzumab and cardiac dysfunction: update on preclinical studies. Semin. Oncol. 30, 49–53 (2003).
Perez, E. A. Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clin. Breast Cancer 8 (Suppl. 3), 114–120 (2008).
Chien, K. R. Herceptin and the heart — a molecular modifier of cardiac failure. N. Engl. J. Med. 354, 789–790 (2006).
Joensuu, H. et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N. Engl. J. Med. 354, 809–820 (2006).
Force, T. & Kerkela, R. Cardiotoxicity of the new cancer therapeutics — mechanisms of, and approaches to, the problem. Drug Discov. Today 13, 778–784 (2008).
Chen, M. H., Kerkela, R. & Force, T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 117, 84–95 (2008).
Crone, S. A. et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature Med. 8, 459–465 (2002).
Kuramochi, Y., Guo, X. & Sawyer, D. B. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J. Mol. Cell Cardiol. 41, 228–235 (2006).
Grazette, L. P. et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J. Am. Coll. Cardiol. 44, 2231–2238 (2004).
Lemmens, K., Doggen, K. & Keulenaer, G. W. Neuregulin-1 and its potential role in the control of cardiac function. Heart Fail. Monit. 5, 119–124 (2008).
Clark, I. A. The advent of the cytokine storm. Immunol. Cell Biol. 85, 271–273 (2007).
Wing, M. Monoclonal antibody first dose cytokine release syndromes — mechanisms and prediction. J. Immunotoxicol. 5, 11–15 (2008).
Plevy, S. et al. A Phase I study of visilzumab, a humanised anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology 133, 1414–1422 (2007).
Wing, M. G., Waldmann, H., Isaacs, J., Compston, D. A. & Hale, G. Ex-vivo whole blood cultures for predicting cytokine-release syndrome: dependence on target antigen and antibody isotype. Ther. Immunol. 2, 183–190 (1995).
Wing, M. G. et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J. Clin. Invest. 98, 2819–2826 (1996).
Winkler, U. et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 94, 2217–2224 (1999).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006). A detailed description of the laboratory events and clinical course after administration of TGN1412 to healthy volunteers: the cytokine storm.
Kenter, M. J. & Cohen, A. F. Establishing risk of human experimentation with drugs: lessons from TGN1412. Lancet 368, 1387–1391 (2006).
Expert Group on Phase One Clinical Trials (Chairman: Professor Gordon W.Duff) Expert Scientific Group on Phase One Clinical Trials: Final Report (The Stationery Office, Norwich, UK, 2006). Report of a UK Expert Group commenting on safety of Phase I studies in the light of the TGN1412 cytokine storm.
Association of the British Pharmaceutical Industry (ABPI), BioIndustry Association (BIA).Early Stage Clinical Trial Taskforce — Joint ABPI/BIA Report. ABPI website [online], (2006).
Muller, P. Y., Milton, M., Lloyd, P., Sims, J. & Brennan, F. R. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr. Opin. Biotechnol. 20, 722–729 (2009).
Beyersdorf, N., Hanke, T., Kerkau, T. & Hunig, T. Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases. Ann. Rheum. Dis. 64 (Suppl. 4), iv91–iv95 (2005).
Beyersdorf, N. et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J. Exp. Med. 202, 445–455 (2005).
Hunig, T. & Dennehy, K. CD28 superagonists: mode of action and therapeutic potential. Immunol. Lett. 100, 21–28 (2005).
Muller, N. et al. A CD28 superagonistic antibody elicits 2 functionally distinct waves of T cell activation in rats. J. Clin. Invest. 118, 1405–1416 (2008).
Hunig, T. Manipulation of regulatory T-cell number and function with CD28-specific monoclonal antibodies. Adv. Immunol. 95, 111–148 (2007).
Schraven, B. & Kalinke, U. CD28 superagonists: what makes the difference in humans? Immunity 28, 591–595 (2008).
Liu, E. H., Siegel, R. M., Harlan, D. M. & O'Shea, J. J. T cell-directed therapies: lessons learned and future prospects. Nature Immunol. 8, 25–30 (2007).
Sharpe, A. H. & Abbas, A. K. T-cell costimulation-biology, therapeutic potential, and challenges. N. Engl. J. Med. 355, 973–975 (2006).
Dayan, C. M. & Wraith, D. C. Preparing for first-in-man studies: the challenges for translational immunology post-TGN1412. Clin. Exp. Immunol. 151, 231–234 (2008).
St Clair, E. W. The calm after the cytokine storm: lessons from the TGN1412 trial. J. Clin. Invest. 118, 1344–1347 (2008).
Hanke, T. Lessons from TGN1412. Lancet 368, 1569–1570 (2006).
Ohresser, M., Olive, D., Vanhove, B. & Watier, H. Risk in drug trials. Lancet 368, 2205–2206 (2006).
Waibler, Z. et al. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS ONE 3, e1708 (2008). In vitro studies on human blood cell signalling with superagonist human CD28-specific mAbs.
Mehrishi, J. N., Szabo, M. & Bakacs, T. Some aspects of the recombinantly expressed humanised superagonist anti-CD28 mAb, TGN1412 trial catastrophe lessons to safeguard mAbs and vaccine trials. Vaccine 25, 3517–3523 (2007).
Yokosuka, T. & Saito, T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol. Rev. 229, 27–40 (2009).
Buysmann, S. et al. Activation and increased expression of adhesion molecules on peripheral blood lymphocytes is a mechanism for the immediate lymphocytopenia after administration of OKT3. Blood 87, 404–411 (1996).
Mourad, G. J. et al. Humanized IgG1 and IgG4 anti-CD4 monoclonal antibodies: effects on lymphocytes in the blood, lymph nodes, and renal allografts in cynomolgus monkeys. Transplantation 65, 632–641 (1998).
Hernandez-Caselles, T. et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J. Leukoc. Biol. 79, 46–58 (2006).
Nguyen, D. H., Hurtado-Ziola, N., Gagneux, P. & Varki, A. Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl Acad. Sci. USA 103, 7765–7770 (2006).
Isaacs, J. D. et al. A therapeutic human IgG4 monoclonal antibody that depletes target cells in humans. Clin. Exp. Immunol. 106, 427–433 (1996).
Avril, T., Attrill, H., Zhang, J., Raper, A. & Crocker, P. R. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem. Soc. Trans. 34, 1024–1027 (2006).
Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nature Rev. Immunol. 7, 255–266 (2007).
Crocker, P. R. & Redelinghuys, P. Siglecs as positive and negative regulators of the immune system. Biochem. Soc. Trans. 36, 1467–1471 (2008).
Gogishvili, T. et al. Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS ONE 4, e4643 (2009).
Perruche, S. et al. Lethal effect of CD3-specific antibody in mice deficient in TGF-β1 by uncontrolled flu-like syndrome. J. Immunol. 183, 953–961 (2009).
Muller, P. Y. & Brennan, F. R. Safety assessment and dose selection for first-in-human clinical trials with immunomodulatory monoclonal antibodies. Clin. Pharmacol. Ther. 85, 247–258 (2009).
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on strategies to identify and mitigate risks for first in human clinical trials with investigational medicinal products. Doc. Ref. EMEA/CHMP/SWP/28367/07. EMA website [online], (2007).
European Medicines Agency. ICH topic M 3 (R2): non-clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals. Note for guidance on non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals (CPMP/ICH/286/95). EMA website [online], (2008).
European Medicines Agency. ICH topic S 6: preclinical safety evaluation of biotechnology-derived pharmaceuticals. Note for guidance on preclinical safety evaluation of biotechnology-derived pharmaceuticals (CPMP/ICH/302/95). EMA website [online], (1998).
Lappin, G. & Garner, R. C. The utility of microdosing over the past 5 years. Expert Opin. Drug Metab. Toxicol. 4, 1499–1506 (2008).
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Position paper on non-clinical safety studies to support clinical trials with a single microdose. CPMP/SWP/2599/02. EMA website [online], (2004).
Liedert, B., Bassus, S., Schneider, C. K., Kalinke, U. & Lower, J. Safety of phase I clinical trials with monoclonal antibodies in Germany — the regulatory requirements viewed in the aftermath of the TGN1412 disaster. Int. J. Clin. Pharmacol. Ther. 45, 1–9 (2007).
Wafelman, A. R. Commentary: symposium report — Development of safe protein therapeutics: pre-clinical, clinical and regulatory issues. Eur. J. Pharm. Sci. 34, 223–225 (2008).
Stebbings, R. et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J. Immunol. 179, 3325–3331 (2007).
Findlay, L. et al. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J. Immunol. Methods 352, 1–12 (2010).References 208 and 209 describe in vitro studies with mAbs and human blood and cell cultures to study the propensity to cause a cytokine storm.
Willmann, J. K., van, B. N., Dinkelborg, L. M. & Gambhir, S. S. Molecular imaging in drug development. Nature Rev. Drug Discov. 7, 591–607 (2008).
Bullen, A. Microscopic imaging techniques for drug discovery. Nature Rev. Drug Discov. 7, 54–67 (2008).
Acknowledgements
We would like to acknowledge the expert assistance of A. Tan with preparation of the figures and generation of the bibliography.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Trevor T. Hansel has received funding for clinical research studies from various pharmaceutical companies (GlaxoSmithKline, Pfizer, Novartis, Institute of Medicinal Molecular Design, Oxagen, Merck) in the past 5 years, and has been given fees for lecturing and attending expert groups (Thomson Reuters, Wyeth, Abbott, AstraZeneca, F. Hoffmann-La Roche, Palau Pharma).
Jane A. Mitchell holds, or has held in the past 5 years, research funds from Hoffmann-La Roche and GlaxoSmithKline. Mitchell has acted as a consultant to a number of pharmaceutical companies including Novartis and NiCOX. Mitchell has acted as an expert witness and received honoraria for guest lectures including those funded by pharmaceutical companies. She is on the scientific advisory board for Antibe Therapeutics.
Harald Kropshofer and Thomas Singer are employees of F. Hoffmann-La Roche, Basel, Switzerland, and holders of equity in this company.
Andrew J. T. George has acted as a consultant to biotechnology companies that are developing antibody therapies, and has shares in one such company.
Related links
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Serum sickness
-
A delayed reaction (generally over 4–10 days) to serum proteins or monoclonal antibodies, consisting of a hypersensitivity reaction with immune-complex generation and vascular damage in the skin, joints and kidneys.
- Tumour lysis syndrome
-
(TLS). A group of metabolic complications that can occur after treatment of cancer, usually lymphomas and leukaemias. It is generally caused by therapy that initiates the acute breakdown of cancer cells. The resultant biochemical abnormalities can cause kidney damage and acute renal failure.
- Cytokine release syndrome
-
(CRS). Also known as cytokine storm. An uncontrolled hypercytokinaemia that results in multiple organ damage and can be associated with monoclonal antibody therapy, infections and cytokine therapy.
- Anaphylaxis
-
A generally immediate and rapid loss of blood pressure (hypotension) due to a type 1 immunoglobulin E-mediated hypersensitivity reaction.
- Thrombocytopaenia
-
A decrease in the number of circulatory platelets in the blood.
- Capillary leak syndrome
-
A leakage of fluid from capillaries into interstitial fluid that results in hypotension, oedema and multiple organ failure due to limited perfusion.
Rights and permissions
About this article
Cite this article
Hansel, T., Kropshofer, H., Singer, T. et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9, 325–338 (2010). https://doi.org/10.1038/nrd3003
Published:
Issue date:
DOI: https://doi.org/10.1038/nrd3003
This article is cited by
-
Monoclonal antibody applications in travel medicine
Tropical Diseases, Travel Medicine and Vaccines (2024)
-
Passive immunotherapy for Alzheimer’s disease: challenges & future directions
Journal of Translational Medicine (2024)
-
Monoclonal antibody infusion reaction with bamlanivimab and etesevimab in a 5-year-old male with coronavirus disease 2019: a case report
Journal of Medical Case Reports (2023)
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)
-
Biodistribution and function of coupled polymer-DNA origami nanostructures
Scientific Reports (2023)