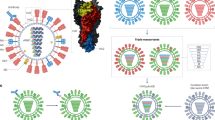
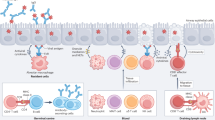
Key Points
-
Seasonal influenza virus vaccines are an effective countermeasure against influenza if the vaccine strains and the circulating viruses are well matched; vaccine efficacy drops sharply if mismatched viruses are circulating.
-
Viruses from the animal reservoir, including H3N2v, H5N1, H5N6, H6N1, H7N3, H7N9 and H10N8, have recently caused morbidity and mortality in humans. Although these viruses are unable to transmit efficiently among humans, the development of pre-pandemic vaccine candidates that could enhance pandemic preparedness is warranted.
-
Pandemic influenza virus vaccines must be produced in a timely manner to effectively reduce the impact of a novel pandemic virus on the global human population. Technological advances such as gene synthesis, reverse genetics and recombinant production systems will facilitate the production of vaccines more rapidly in response to future influenza pandemics.
-
Novel human monoclonal antibody technology has helped provide a better understanding of the humoral (crossreactive) immune responses against the influenza virus surface glycoproteins haemagglutinin and neuraminidase.
-
Glycosylation of haemagglutinin and neuraminidase has a role in the immunogenicity of influenza virus vaccines and vaccine candidates.
-
Broadly protective antibodies against the haemagglutinin stalk domain and neuraminidase guide the design of novel, broadly protective vaccines. Novel influenza virus vaccine candidates that induce broad or universal influenza virus coverage are currently in preclinical and clinical development.
-
Broadly protective or universal influenza virus vaccines could abolish the need for annual reformulation and re-administration of seasonal influenza virus vaccines and could improve our pandemic preparedness.
Abstract
Influenza virus infections are a major public health concern and cause significant morbidity and mortality worldwide. Current influenza virus vaccines are an effective countermeasure against infection but need to be reformulated almost every year owing to antigenic drift. Furthermore, these vaccines do not protect against novel pandemic strains, and the timely production of pandemic vaccines remains problematic because of the limitations of current technology. Several improvements have been made recently to enhance immune protection induced by seasonal and pandemic vaccines, and to speed up production in case of a pandemic. Importantly, vaccine constructs that induce broad or even universal influenza virus protection are currently in preclinical and clinical development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
06 March 2015
The citation number for reference 41, a World Health Organization monthly risk asssesment summary, was missing in the article text and reference list. This has been corrected in the online version. The statement at the end of the legend for Figure 1, related to the permission to adapt a figure in reference 227, has also been modified.
References
World Health Organization. Influenza (seasonal) fact sheet. World Health Organization [online], (2014).
Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 21, 1776–1779 (2003).
Palese, P. Influenza: old and new threats. Nature Med. 10, S82–S87 (2004).
Johnson, N. P. & Mueller, J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 76, 105–115 (2002).
Palese, P. & Wang, T. T. Why do influenza virus subtypes die out? A hypothesis. MBio 2, e00150-11 (2011).
Racaniello, V. Pandemic influenza vaccine was too late in 2009. Virology Blog [online], (2010).
Krammer, F. & Palese, P. Universal influenza virus vaccines: need for clinical trials. Nature Immunol. 15, 3–5 (2014).
Francis, T., Salk, J. E., Pearson, H. E. & Brown, P. N. Protective effect of vaccination against induced influenza A. J. Clin. Invest. 24, 536–546 (1945).
Salk, J. E., Pearson, H. E., Brown, P. N. & Francis, T. Protective effect of vaccination against induced influenza B. J. Clin. Invest. 24, 547–553 (1945).
Salk, J. E. & Suriano, P. C. Importance of antigenic composition of influenza virus vaccine in protecting against the natural disease; observations during the winter of 1947–1948. Am. J. Public Health Nations Health 39, 345–355 (1949).
Payne, A. M. The influenza programme of WHO. Bull. World Health Organ. 8, 755–774 (1953).
Allison, J. E., Glezen, W. P., Taber, L. H., Paredes, A. & Webster, R. G. Reactogenicity and immunogenicity of bivalent influenza A and monovalent influenza B virus vaccines in high-risk children. J. Infect. Dis. 136, S672–S676 (1977).
Davenport, F. M. et al. Comparisons of serologic and febrile responses in humans to vaccination with influenza A viruses or their hemagglutinins. J. Lab. Clin. Med. 63, 5–13 (1964).
Jin, H. & Subbarao, K. Live attenuated influenza vaccine. Curr. Top. Microbiol. Immunol. 386, 181–204 (2014).
Maassab, H. F. Adaptation and growth characteristics of influenza virus at 25 °C. Nature 213, 612–614 (1967).
Alexandrova, G. I. et al. Study of live recombinant cold-adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine 4, 114–118 (1986).
Tricco, A. C. et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 11, 153 (2013).
Steinhoff, M. C. et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ 184, 645–653 (2012).
Sheffield, J. S. et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet. Gynecol. 120, 532–537 (2012).
Beyer, W. E. et al. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine 31, 6030–6033 (2013).
Ohmit, S. E. et al. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 56, 1363–1369 (2013).
Kissling, E. et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case–control study. Euro Surveill. 18, 20390 (2013).
Clark, A. et al. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunity. J. Hyg. (Lond.) 90, 361–370 (1983).
de Jong, J. C., Beyer, W. E., Palache, A. M., Rimmelzwaan, G. F. & Osterhaus, A. D. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 61, 94–99 (2000).
DiazGranados, C. A. et al. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine 31, 861–866 (2013).
DiazGranados, C. A. et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 371, 635–645 (2014).
O'Hagan, D. T., Ott, G. S., Nest, G. V., Rappuoli, R. & Giudice, G. D. The history of MF59® adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines 12, 13–30 (2013).
Del Giudice, G. & Rappuoli, R. Inactivated and adjuvanted influenza vaccines. Curr. Top. Microbiol. Immunol. 386, 151–180 (2014).
Ledgerwood, J. E. AS03-adjuvanted influenza vaccine in elderly people. Lancet Infect. Dis. 13, 466–467 (2013).
Tinoco, J. C. et al. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: a phase III, randomized trial. Vaccine 32, 1480–1487 (2014).
Jain, V. K. et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N. Engl. J. Med. 369, 2481–2491 (2013).
Esposito, S. & Principi, N. Vaccine for prevention of influenza in children. N. Engl. J. Med. 370, 1167 (2014).
Wei, C. J. et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064 (2010).
Wei, C. J. et al. Elicitation of broadly neutralizing influenza antibodies in animals with previous influenza exposure. Sci. Transl. Med. 4, 147ra114 (2012).
Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013).
Centers for Disease Control and Prevention. Table. Influenza vaccines — United States, 2014–15 influenza season. Centers for Disease Control and Prevention [online], (2014).
US Food and Drug Administration. FDA approves new seasonal influenza vaccine made using novel technology. US Food and Drug Administration [online], (2013).
Claas, E. C. et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–477 (1998).
Vijaykrishna, D. et al. Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathog. 4, e1000161 (2008).
Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840–1842 (2001).
World Health Organization. The WHO Influenza Monthly Risk Assessment Summaries. World Health Organization [online], (2015).
Wang, T. T., Parides, M. K. & Palese, P. Seroevidence for H5N1 influenza infections in humans: meta-analysis. Science 335, 1463 (2012).
Garten, R. et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325, 197–201 (2009).
Gao, R. et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897 (2013).
Wei, S. H. et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir. Med. 1, 771–778 (2013).
García-Sastre, A. & Schmolke, M. Avian influenza A H10N8 — a virus on the verge? Lancet 383, 676–677 (2014).
Chen, H. et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 383, 714–721 (2014).
Park, M. World's first H5N6 bird flu death reported in China. CNN [online], (2014).
Centers for Disease Control and Prevention (CDC). Notes from the field: outbreak of influenza A (H3N2) virus among persons and swine at a county fair — Indiana, July 2012. MMWR Morb. Mortal. Wkly Rep. 61, 561 (2012).
Lopez-Martinez, I. et al. Highly pathogenic avian influenza A(H7N3) virus in poultry workers, Mexico, 2012. Emerg. Infect. Dis. 19, 1531–1534 (2013).
Anthony, S. J. et al. Emergence of fatal avian influenza in New England harbor seals. MBio 3, e00166-12 (2012).
Zohari, S., Neimanis, A., Harkonen, T., Moraeus, C. & Valarcher, J. Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill. 19, 20967 (2014).
[No authors listed.] Avian influenza outbreak in Yorkshire: strain identified as H5N8. Vet. Rec. 175, 495–496 (2014).
Krammer, F. & Cox, R. J. The emergence of H7N9 viruses: a chance to redefine correlates of protection for influenza virus vaccines. Expert Rev. Vaccines 12, 1369–1372 (2013).
Cox, R. J. et al. A phase I clinical trial of a PER.C6® cell grown influenza H7 virus vaccine. Vaccine 27, 1889–1897 (2009).
Couch, R. B. et al. Evaluations for in vitro correlates of immunogenicity of inactivated influenza a H5, H7 and H9 vaccines in humans. PLoS ONE 7, e50830 (2012).
Couch, R. B., Patel, S. M., Wade-Bowers, C. L. & Niño, D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS ONE 7, e49704 (2012).
Kistner, O. et al. Cell culture (Vero) derived whole virus (H5N1) vaccine based on wild-type virus strain induces cross-protective immune responses. Vaccine 25, 6028–6036 (2007).
Baz, M., Luke, C. J., Cheng, X., Jin, H. & Subbarao, K. H5N1 vaccines in humans. Virus Res. 178, 78–98 (2013).
Belshe, R. B. et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA 312, 1420–1428 (2014).
Mulligan, M. J. et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 312, 1409–1419 (2014).
Krammer, F. et al. An H7N1 influenza virus vaccine induces broadly reactive antibody responses against H7N9 in humans. Clin. Vaccine Immunol. 21, 1153–1163 (2014).
Ellebedy, A. H. et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl Acad. Sci. USA 111, 13133–13138 (2014).
Nachbagauer, R. et al. Induction of broadly-reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J. Virol. 88, 13260–13268 (2014).
Karron, R. A. et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 27, 4953–4960 (2009).
Talaat, K. R. et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine 27, 3744–3753 (2009).
Talaat, K. R. et al. An open label phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine 29, 3144–3148 (2011).
Talaat, K. R. et al. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respir. Viruses 7, 66–73 (2013).
Rudenko, L. et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PLoS ONE 9, e87962 (2014).
Rudenko, L., Isakova-Sivak, I. & Donina, S. H7N3 live attenuated influenza vaccine has a potential to protect against new H7N9 avian influenza virus. Vaccine 31, 4702–4705 (2013).
Rudenko, L. et al. Safety and immunogenicity of live attenuated influenza reassortant H5 vaccine (phase I–II clinical trials). Influenza Other Respir. Viruses 2, 203–209 (2008).
Matsuoka, Y. et al. African green monkeys recapitulate the clinical experience with replication of live attenuated pandemic influenza virus vaccine candidates. J. Virol. 88, 8139–8152 (2014).
Min, J. Y. et al. A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J. Virol. 84, 11950–11960 (2010).
Steel, J. et al. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J. Virol. 83, 1742–1753 (2009).
de Graaf, M. & Fouchier, R. A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 33, 823–841 (2014).
Ledgerwood, J. E. et al. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J. Infect. Dis. 208, 418–422 (2013).
Talaat, K. R. et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J. Infect. Dis. 209, 1860–1869 (2014).
Luke, C. J. & Subbarao, K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert Rev. Vaccines 13, 873–883 (2014).
Kistner, O. et al. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine 16, 960–968 (1998).
Dormitzer, P. R. Rapid production of synthetic influenza vaccines. Curr. Top. Microbiol. Immunol. 386, 237–273 (2015).
Fodor, E. et al. Rescue of influenza A virus from recombinant DNA. J. Virol. 73, 9679–9682 (1999).
Cox, M. M. Recombinant protein vaccines produced in insect cells. Vaccine 30, 1759–1766 (2012).
Krammer, F. & Grabherr, R. Alternative influenza vaccines made by insect cells. Trends Mol. Med. 16, 313–320 (2010).
Jul-Larsen, Å. et al. The human potential of a recombinant pandemic influenza vaccine produced in tobacco plants. Hum. Vaccin. Immunother. 8, 653–661 (2012).
Aguilar-Yáñez, J. M. et al. An influenza A/H1N1/2009 hemagglutinin vaccine produced in Escherichia coli. PLoS ONE 5, e11694 (2010).
Taylor, D. N. et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza–flagellin fusion vaccine with improved safety and immune response. Vaccine 30, 5761–5769 (2012).
Shi, S. H. et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarum NC8 expressing hemagglutinin in BALB/c mice. Virology 464–465, 166–176 (2014).
Bayne, A. C. et al. Vaccination against influenza with recombinant hemagglutinin expressed by Schizochytrium sp. confers protective immunity. PLoS ONE 8, e61790 (2013).
Saelens, X. et al. Protection of mice against a lethal influenza virus challenge after immunization with yeast-derived secreted influenza virus hemagglutinin. Eur. J. Biochem. 260, 166–175 (1999).
Murugan, S. et al. Recombinant haemagglutinin protein of highly pathogenic avian influenza A (H5N1) virus expressed in Pichia pastoris elicits a neutralizing antibody response in mice. J. Virol. Methods 187, 20–25 (2013).
Welsh, J. P., Lu, Y., He, X. S., Greenberg, H. B. & Swartz, J. R. Cell-free production of trimeric influenza hemagglutinin head domain proteins as vaccine antigens. Biotechnol. Bioeng. 109, 2962–2969 (2012).
DuBois, R. M. et al. The receptor-binding domain of influenza virus hemagglutinin produced in Escherichia coli folds into its native, immunogenic structure. J. Virol. 85, 865–872 (2011).
Khurana, S. et al. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies in humans that preferentially bind to oligomeric form of influenza hemagglutinin. J. Virol. 85, 10945–10954 (2011).
López-Macías, C. et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 29, 7826–7834 (2011).
Krammer, F. et al. Swine-origin pandemic H1N1 influenza virus-like particles produced in insect cells induce hemagglutination inhibiting antibodies in BALB/c mice. Biotechnol. J. 5, 17–23 (2010).
Smith, G. E. et al. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine 31, 4305–4313 (2013).
Klausberger, M. et al. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine 32, 355–362 (2014).
Margine, I., Martinez-Gil, L., Chou, Y. Y. & Krammer, F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS ONE 7, e51559 (2012).
D'Aoust, M. et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 6, 930–940 (2008).
D'Aoust, M. A. et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol. J. 8, 607–619 (2010).
Fries, L. F., Smith, G. E. & Glenn, G. M. A recombinant viruslike particle influenza A (H7N9) vaccine. N. Engl. J. Med. 369, 2564–2566 (2013).
Landry, N. et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 5, e15559 (2010).
Ledgerwood, J. E. et al. Influenza virus H5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin. Vaccine Immunol. 19, 1792–1797 (2012).
Tripp, R. A. & Tompkins, S. M. Virus-vectored influenza virus vaccines. Viruses 6, 3055–3079 (2014).
Rimmelzwaan, G. F. & Sutter, G. Candidate influenza vaccines based on recombinant modified vaccinia virus Ankara. Expert Rev. Vaccines 8, 447–454 (2009).
Kreijtz, J. H. et al. Evaluation of a modified vaccinia virus Ankara (MVA)-based candidate pandemic influenza A/H1N1 vaccine in the ferret model. J. Gen. Virol. 91, 2745–2752 (2010).
Altenburg, A. F. et al. Modified vaccinia virus Ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases. Viruses 6, 2735–2761 (2014).
Kreijtz, J. H. et al. Recombinant modified vaccinia virus Ankara expressing the hemagglutinin gene confers protection against homologous and heterologous H5N1 influenza virus infections in macaques. J. Infect. Dis. 199, 405–413 (2009).
Kreijtz, J. H. et al. A single immunization with an MVA-based influenza virus H7 vaccine affords protection in the H7N9 pneumonia ferret model. J. Infect. Dis. http://dx.doi.org/10.1093/infdis/jiu528 (2014).
Prabakaran, M. et al. Progress toward a universal H5N1 vaccine: a recombinant modified vaccinia virus Ankara-expressing trivalent hemagglutinin vaccine. PLoS ONE 9, e107316 (2014).
Kreijtz, J. H. et al. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect. Dis. 14, 1196–1207 (2014).
Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 (2011).
Wrammert, J. et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453, 667–671 (2008).
Moody, M. A. et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS ONE 6, e25797 (2011).
Throsby, M. et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 3, e3942 (2008).
Sui, J. et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nature Struct. Mol. Biol. 16, 265–273 (2009).
Corti, D. et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–1673 (2010).
Okuno, Y., Isegawa, Y., Sasao, F. & Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67, 2552–2558 (1993).
Kashyap, A. K. et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl Acad. Sci. USA 105, 5986–5991 (2008).
Wan, H. et al. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J. Virol. 87, 9290–9300 (2013).
Krause, J. C. et al. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 85, 10905–10908 (2011).
Krause, J. C. et al. Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J. Virol. 86, 6334–6340 (2012).
Ekiert, D. C. et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012).
Whittle, J. R. et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl Acad. Sci. USA 108, 14216–14221 (2011).
Ohshima, N. et al. Naturally occurring antibodies in a human can neutralize a broad spectrum of influenza strains including H3, H1, H2 and H5. J. Virol. 85, 11048–11057 (2011).
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009).
Friesen, R. H. et al. A common solution to group 2 influenza virus neutralization. Proc. Natl Acad. Sci. USA 111, 445–450 (2014).
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011).
Tan, G. S. et al. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J. Virol. 88, 13580–13592 (2014).
Tan, G. S. et al. A pan-h1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 86, 6179–6188 (2012).
Wang, T. T. et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6, e1000796 (2010).
Dreyfus, C. et al. Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011).
Nakamura, G. et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14, 93–103 (2013).
Krammer, F. et al. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS ONE 7, e43603 (2012).
Harris, A. K. et al. Structure and accessibility of HA trimers on intact 2009 H1N1 pandemic influenza virus to stem region-specific neutralizing antibodies. Proc. Natl Acad. Sci. USA 110, 4592–4597 (2013).
Brandenburg, B. et al. Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS ONE 8, e80034 (2013).
Terajima, M. et al. Complement-dependent lysis of influenza A virus-infected cells by broadly cross-reactive human monoclonal antibodies. J. Virol. 85, 13463–13467 (2011).
Dilillo, D. J., Tan, G. S., Palese, P. & Ravetch, J. V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nature Med. 20, 143–151 (2014).
Jegaskanda, S., Weinfurter, J. T., Friedrich, T. C. & Kent, S. J. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J. Virol. 87, 5512–5522 (2013).
Jegaskanda, S. et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J. Immunol. 190, 1837–1848 (2013).
Jegaskanda, S., Reading, P. C. & Kent, S. J. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J. Immunol. 193, 469–475 (2014).
Margine, I. et al. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87, 4728–4737 (2013).
Krammer, F., Pica, N., Hai, R., Tan, G. S. & Palese, P. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J. Virol. 86, 10302–10307 (2012).
Miller, M. S. et al. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci. Transl. Med. 5, 198ra107 (2013).
Pica, N. et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl Acad. Sci. USA 109, 2573–2578 (2012).
Thomson, C. A. et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 3, 87 (2012).
Miller, M. S. et al. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J. Infect. Dis. 207, 98–105 (2013).
Sangster, M. Y. et al. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clin. Vaccine Immunol. 20, 867–876 (2013).
Whittle, J. R. et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol. 88, 4047–4057 (2014).
Hensley, S. E. Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Curr. Opin. Virol. 8, 85–89 (2014).
Li, Y. et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J. Exp. Med. 210, 1493–1500 (2013).
Wohlbold, T. J. & Krammer, F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6, 2465–2494 (2014).
Doyle, T. M. et al. A monoclonal antibody targeting a highly conserved epitope in influenza B neuraminidase provides protection against drug resistant strains. Biochem. Biophys. Res. Commun. 441, 226–229 (2013).
Doyle, T. M. et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral Res. 100, 567–574 (2013).
Tate, M. D. et al. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6, 1294–1316 (2014).
Medina, R. A. et al. Glycosylations in the globular head of the hemagglutinin protein modulate the virulence and antigenic properties of the H1N1 influenza viruses. Sci. Transl. Med. 5, 187ra70 (2013).
Wang, C. C. et al. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl Acad. Sci. USA 106, 18137–18142 (2009).
Chen, J. R. et al. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc. Natl Acad. Sci. USA 111, 2476–2481 (2014).
Magadán, J. G. et al. Biogenesis of influenza A virus hemagglutinin cross-protective stem epitopes. PLoS Pathog. 10, e1004204 (2014).
An, Y. et al. Comparative glycomics analysis of influenza hemagglutinin (H5N1) produced in vaccine relevant cell platforms. J. Proteome Res. 12, 3707–3720 (2013).
Palmberger, D., Ashjaei, K., Strell, S., Hoffmann-Sommergruber, K. & Grabherr, R. Minimizing fucosylation in insect cell-derived glycoproteins reduces binding to IgE antibodies from the sera of patients with allergy. Biotechnol. J. 9, 1206–1214 (2014).
Eggink, D., Goff, P. H. & Palese, P. Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 88, 699–704 (2014).
Lin, S. C., Lin, Y. F., Chong, P. & Wu, S. C. Broader neutralizing antibodies against H5N1 viruses using prime-boost immunization of hyperglycosylated hemagglutinin DNA and virus-like particles. PLoS ONE 7, e39075 (2012).
Lin, S. C., Liu, W. C., Jan, J. T. & Wu, S. C. Glycan masking of hemagglutinin for adenovirus vector and recombinant protein immunizations elicits broadly neutralizing antibodies against H5N1 avian influenza viruses. PLoS ONE 9, e92822 (2014).
Graves, P. N., Schulman, J. L., Young, J. F. & Palese, P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology 126, 106–116 (1983).
Sagawa, H., Ohshima, A., Kato, I., Okuno, Y. & Isegawa, Y. The immunological activity of a deletion mutant of influenza virus haemagglutinin lacking the globular region. J. Gen. Virol. 77, 1483–1487 (1996).
Steel, J. et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 1, e00018-10 (2010).
Bommakanti, G. et al. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl Acad. Sci. USA 107, 13701–13706 (2010).
Bommakanti, G. et al. Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J. Virol. 86, 13434–13444 (2012).
Mallajosyula, V. V. et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl Acad. Sci. USA 111, E2514–E2523 (2014).
Lu, Y., Welsh, J. P. & Swartz, J. R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl Acad. Sci. USA 111, 125–130 (2014).
Staneková, Z. et al. Heterosubtypic protection against influenza A induced by adenylate cyclase toxoids delivering conserved HA2 subunit of hemagglutinin. Antiviral Res. 97, 24–35 (2013).
Stropkovská, A. et al. Broadly cross-reactive monoclonal antibodies against HA2 glycopeptide of influenza A virus hemagglutinin of H3 subtype reduce replication of influenza A viruses of human and avian origin. Acta Virol. 53, 15–20 (2009).
Hai, R. et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86, 5774–5781 (2012).
Krammer, F. & Palese, P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr. Opin. Virol. 3, 521–530 (2013).
Krammer, F. et al. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J. Virol. 88, 3432–3442 (2014).
Krammer, F., Pica, N., Hai, R., Margine, I. & Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87, 6542–6550 (2013).
Krammer, F. et al. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 88, 2340–2343 (2014).
Margine, I. et al. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. 87, 10435–10446 (2013).
Goff, P. H. et al. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS ONE 8, e79194 (2013).
Weaver, E. A., Rubrum, A. M., Webby, R. J. & Barry, M. A. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS ONE 6, e18314 (2011).
Kirchenbaum, G. A. & Ross, T. M. Eliciting broadly protective antibody responses against influenza. Curr. Opin. Immunol. 28, 71–76 (2014).
Ducatez, M. F. et al. Feasibility of reconstructed ancestral H5N1 influenza viruses for cross-clade protective vaccine development. Proc. Natl Acad. Sci. USA 108, 349–354 (2011).
Giles, B. M. & Ross, T. M. Computationally optimized antigens to overcome influenza viral diversity. Expert Rev. Vaccines 11, 267–269 (2012).
Giles, B. M., Bissel, S. J., Dealmeida, D. R., Wiley, C. A. & Ross, T. M. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5N1 virus-like particle vaccines. Clin. Vaccine Immunol. 19, 128–139 (2012).
Giles, B. M. & Ross, T. M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 29, 3043–3054 (2011).
Giles, B. M. et al. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5N1 infection. J. Infect. Dis. 205, 1562–1570 (2012).
Kilbourne, E. D., Johansson, B. E. & Grajower, B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl Acad. Sci. USA 87, 786–790 (1990).
Abed, Y., Hardy, I., Li, Y. & Boivin, G. Divergent evolution of hemagglutinin and neuraminidase genes in recent influenza A:H3N2 viruses isolated in Canada. J. Med. Virol. 67, 589–595 (2002).
Westgeest, K. B. et al. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J. Gen. Virol. 93, 1996–2007 (2012).
Sultana, I. et al. Stability of neuraminidase in inactivated influenza vaccines. Vaccine 32, 2225–2230 (2014).
Couch, R. B., Kasel, J. A., Gerin, J. L., Schulman, J. L. & Kilbourne, E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 129, 411–420 (1974).
Johansson, B. E., Moran, T. M. & Kilbourne, E. D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl Acad. Sci. USA 84, 6869–6873 (1987).
Johansson, B. E. & Kilbourne, E. D. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J. Virol. 67, 5721–5723 (1993).
Johansson, B. E. & Kilbourne, E. D. Immunization with purified N1 and N2 influenza virus neuraminidases demonstrates cross-reactivity without antigenic competition. Proc. Natl Acad. Sci. USA 91, 2358–2361 (1994).
Kilbourne, E. D. et al. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine 13, 1799–1803 (1995).
Easterbrook, J. D. et al. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432, 39–44 (2012).
Kilbourne, E. D., Cerini, C. P., Khan, M. W., Mitchell, J. W. & Ogra, P. L. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J. Immunol. 138, 3010–3013 (1987).
Schotsaert, M., De Filette, M., Fiers, W. & Saelens, X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev. Vaccines 8, 499–508 (2009).
Neirynck, S. et al. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nature Med. 5, 1157–1163 (1999).
De Filette, M. et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J. Biol. Chem. 283, 11382–11387 (2008).
Wang, L. et al. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine 10, 473–482 (2014).
Huleatt, J. et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26, 201–214 (2008).
De Filette, M. et al. Universal influenza A vaccine: optimization of M2-based constructs. Virology 337, 149–161 (2005).
Ebrahimi, S. M., Dabaghian, M., Tebianian, M. & Jazi, M. H. In contrast to conventional inactivated influenza vaccines, 4xM2e. HSP70c fusion protein fully protected mice against lethal dose of H1, H3 and H9 influenza A isolates circulating in Iran. Virology 430, 63–72 (2012).
El Bakkouri, K. et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J. Immunol. 186, 1022–1031 (2011).
Ramos, E. L. et al. Efficacy and safety of treatment with an anti-M2e monoclonal antibody in experimental human influenza. J. Infect. Dis. http://dx.doi.org/10.1093/infdis/jiu539 (2014).
Berthoud, T. K. et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA–NP+M1. Clin. Infect. Dis. 52, 1–7 (2011).
Antrobus, R. D. et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA–NP+M1 in adults aged over 50 years. PLoS ONE 7, e48322 (2012).
Lillie, P. J. et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA–NP+M1, in humans. Clin. Infect. Dis. 55, 19–25 (2012).
Mullarkey, C. E. et al. Improved adjuvanting of seasonal influenza vaccines: preclinical studies of MVA–NP+M1 coadministration with inactivated influenza vaccine. Eur. J. Immunol. 43, 1940–1952 (2013).
Antrobus, R. D. et al. Coadministration of seasonal influenza vaccine and MVA–NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol. Ther. 22, 233–238 (2014).
Lambe, T. et al. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci. Rep. 3, 1443 (2013).
Valkenburg, S. A. et al. IL-15 adjuvanted multivalent vaccinia-based universal influenza vaccine requires CD4+ T cells for heterosubtypic protection. Proc. Natl Acad. Sci. USA 111, 5676–5681 (2014).
Baker, S. F. et al. Protection against lethal influenza with a viral mimic. J. Virol. 87, 8591–8605 (2013).
Yang, C., Skiena, S., Futcher, B., Mueller, S. & Wimmer, E. Deliberate reduction of hemagglutinin and neuraminidase expression of influenza virus leads to an ultraprotective live vaccine in mice. Proc. Natl Acad. Sci. USA 110, 9481–9486 (2013).
Powell, T. J., Silk, J. D., Sharps, J., Fodor, E. & Townsend, A. R. Pseudotyped influenza A virus as a vaccine for the induction of heterotypic immunity. J. Virol. 86, 13397–13406 (2012).
Wang, T. T. et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl Acad. Sci. USA 107, 18979–18984 (2010).
Staneková, Z. et al. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 55, 61–67 (2011).
Janulíková, J., Staneková, Z., Mucha, V., Kostolanský, F. & Varecková, E. Two distinct regions of HA2 glycopolypeptide of influenza virus hemagglutinin elicit cross-protective immunity against influenza. Acta Virol. 56, 169–176 (2012).
Atsmon, J. et al. Safety and immunogenicity of multimeric-001—a novel universal influenza vaccine. J. Clin. Immunol. 32, 595–603 (2012).
Atsmon, J. et al. Priming by a novel universal influenza vaccine (multimeric-001)—a gateway for improving immune response in the elderly population. Vaccine 32, 5816–5823 (2014).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nature Med. 19, 1305–1312 (2013).
Hillaire, M. L. et al. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J. Gen. Virol. 92, 2339–2349 (2011).
van de Sandt, C. E. et al. Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus. J. Virol. 88, 1684–1693 (2013).
World Health Organization. Pandemic influenza vaccine manufacturing process and timeline. World Health Organization [online], (2009).
Lee, P. S. et al. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nature Commun. 5, 3614 (2014).
Li, Q. et al. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nature Struct. Mol. Biol. 17, 1266–1268 (2010).
Bohne-Lang, A. & von der Lieth, C. W. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 33, W214–W219 (2005).
Gamblin, S. et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303, 1838–1842 (2004).
Xu, X., Zhu, X., Dwek, R. A., Stevens, J. & Wilson, I. A. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J. Virol. 82, 10493–10501 (2008).
Acknowledgements
The authors thank T. J. Wohlbold for help with GlyProt and PyMOL. Research in the Krammer laboratory is supported by a US National Institutes of Health (NIH) Centres for Excellence in Influenza Research and Surveillance (CEIRS) contract (HHSN272201400008C). P.P. is supported by an NIH CEIRS contract (HHSN272201400008C) and by NIH grants (U19 AI109946 and P01 AI097092).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The Icahn School of Medicine at Mount Sinai has filed several patents regarding influenza virus vaccine constructs.
Related links
Glossary
- Antigenic drift
-
A mechanism by which influenza viruses escape from human 'herd immunity'. The RNA-dependent RNA polymerase of influenza viruses is relatively error prone and has no proofreading mechanism, resulting in a high frequency of point mutations. Immunologic pressure in the human population then selects for mutants that can escape from this herd immunity. The globular head domain of haemagglutinin is — owing to its immuno-dominance and high plasticity — most affected by antigenic drift.
- Haemagglutinin
-
(HA). A homotrimeric viral surface glycoprotein that mediates the attachment of influenza viruses to cells by binding to sialic acids on glycan structures of cellular receptors. Haemagglutinin also mediates the fusion of viral and endosomal membranes, which causes the release of the viral genome into the cytosol. Haemagglutinin is the major antigen of the virus.
- Neuraminidase
-
(NA). A viral homotetrameric viral surface glycoprotein with sialidase activity. Neuraminidase helps transport the virus trough mucosal surfaces and mediates the release of budding viruses from the cell surface.
- Whole-virus inactivated vaccines
-
Whole-virus inactivated vaccines are based on intact virions that have been chemically (for example, with formalin or β-propiolactone) or physically (for example, with ultraviolet light) inactivated. Treatment of these virions with detergent leads to split vaccines. Further (partial) purification of the haemagglutinin and neuraminidase of viruses results in subunit vaccines.
- Haemagglutination inhibition
-
(HI). Haemagglutination activity is the standard correlate of protection used for influenza virus vaccines, and haemagglutination inhibition describes the ability of antibodies to block the binding of the haemagglutinin globular head domain to cellular receptors. Stalk-reactive antibodies are generally haemagglutination inhibition negative.
- Neuraminidase inhibition
-
(NI). NI describes the ability of antibodies to block the sialidase function of neuraminidase.
Rights and permissions
About this article
Cite this article
Krammer, F., Palese, P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14, 167–182 (2015). https://doi.org/10.1038/nrd4529
Published:
Issue date:
DOI: https://doi.org/10.1038/nrd4529
This article is cited by
-
A structure and knowledge-based combinatorial approach to engineering universal scFv antibodies against influenza M2 protein
Journal of Biomedical Science (2023)
-
Potential cross-species transmission of highly pathogenic avian influenza H5 subtype (HPAI H5) viruses to humans calls for the development of H5-specific and universal influenza vaccines
Cell Discovery (2023)
-
Trends in nano-platforms for the treatment of viral infectious diseases
Korean Journal of Chemical Engineering (2023)
-
Enhanced passive safety surveillance of a quadrivalent inactivated split virion influenza vaccine in Finland during the influenza season 2020/21
BMC Public Health (2022)
-
Generation of a live attenuated influenza A vaccine by proteolysis targeting
Nature Biotechnology (2022)