Key Points
-
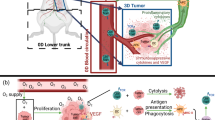
For a tumour to grow beyond a certain size, it must develop a network of blood vessels to supply nutrients and oxygen and to remove waste products.
-
Advances in the understanding of this process of tumour angiogenesis have led to the development of many drugs that target the different steps that are involved, more than 30 of which have entered clinical trials. However, so far, none has been approved, and there have been several prominent failures, which might be because clinical approaches that were established for traditional cytotoxic agents are inappropriate for angiogenic modulators.
-
The usual clinical development of a cytotoxic agent is based on the following concepts: first, that the agent is associated with dose-dependent toxicity; second, that there is an upper limit for dose escalation, which is defined as the dose-limiting toxicity (DLT); third, that the maximum-tolerated dose (MTD) has a higher probability of shrinking tumours (defined as objective remission) and improving palliation of symptoms; and finally, that the agent or combination regimens that are associated with tumour shrinkage might prolong survival.
-
By contrast, in early Phase I/II trials, angiogenic modulators have shown modest toxic effects and are mainly cytostatic, slowing or stopping the tumour growth and the development of metastases without producing an objective remission.
-
It seems clear that the end points for dose-defining trials (Phase I) and efficacy trials (Phase II) should be reconsidered. We strongly recommend the extensive use of correlative studies in the early phases of drug development to establish surrogate biomarkers for use in efficacy trials. Novel Phase II trial designs should be considered to address issues such as the low probability of an objective measurable response with angiogenic modulators.
-
Imaging studies could have a key role in assessing the efficacy of treatments. Various imaging modalities, such as magnetic resonance imaging, ultrasonography, positron emission tomography and computed tomography, can be selected for this purpose; the choice of imaging studies should be based on an accurate evaluation of the novel agents in preclinical models.
-
Finally, careful selection of the clinical setting for the investigation (for example, tumour type and stage of disease) must be carried out before expensive, definitive Phase III clinical trials are initiated.
Abstract
Angiogenesis — the formation of new blood vessels — is essential for tumour progression and metastasis. Consequently, the modulation of tumour angiogenesis using novel agents has become a highly active area of investigation in cancer research, from the bench to the clinic. However, the great therapeutic potential of these agents has yet to be realized, which could, in part, be because the traditional strategies that are used in clinical trials for anticancer therapies are not appropriate for assessing the efficacy of agents that modulate angiogenesis. Here, we discuss methods for monitoring the biological activity of angiogenic modulators, and innovative approaches to trial design that might facilitate the integration of these agents into anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000).
Folkman, J. Tumour angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Skobe, M. et al. Halting angiogenesis suppresses carcinoma cell invasion. Nature Med. 3, 1222–1227 (1997).
Zhang, H. T. et al. Enhancement of tumour growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J. Natl Cancer Inst. 87, 213–219 (1995).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2001).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Bouck, N., Stellmach, V. & Hsu, S. C. How tumours become angiogenic. Adv. Cancer Res. 69, 135–174 (1996).
Robinson, C. J. et al. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 114, 853–865 (2001).
Hiraoka, N. et al. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 91, 365–377 (1998).
Levine, H. A. et al. A mathematical model for the roles of pericytes and macrophages in the initiation of angiogenesis. The role of protease inhibitors in preventing angiogenesis. Mathem. Biosc. 168, 77–115 (2000).
Sturzebecher, J. et al. 3-Amidinophenylalanine-based inhibitors of urokinase. Bioorg. Med. Chem. Lett. 9, 3147–3152 (1999).
Presta, L. G. et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumours and other disorders. Cancer Res. 57, 4593–4599 (1997).This paper is an extremely interesting example of the progress of an angiogenic modulator in more advanced clinical development.
Shaheen, R. M. et al. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumour and endothelial cell apoptosis. Cancer Res. 59, 5412–5416 (1999).
Oshika, Y. et al. Ribozyme approach to downregulate vascular endothelial growth factor (VEGF) 189 expression in non-small cell lung cancer (NSCLC). Eur. J. Cancer 36, 2390–2396 (2000).
Westphal, J. R. et al. Angiostatin generation by human tumour cell lines: involvement of plasminogen activators. Int. J. Cancer 86, 760–767 (2000).
Huang, X. et al. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumour effect. Cancer Res. 61, 478–481 (2001).
Fotsis, T. et al. The endogenous oestrogen metabolite 2-methoxyestradiol inhibits angiogenesis and suppresses tumour growth. Nature 368, 237–239 (1994).
Pasqualini, R. et al. Aminopeptidase N is a receptor for tumour-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 60, 722–727 (2000).
Chen, J., Baskerville, C., Han, Q., Pan, Z. K., & Huang, S. αv-Integrin, p38 mitogen-activated protein kinase, and urokinase plasminogen activator are functionally linked in invasive breast cancer cells. J. Biol. Chem. 276, 47901–47905 (2001).
O-Charoenrat, P., Rhys-Evans, P., Modjtahedi, H. & Eccles, S. A. Vascular endothelial growth factor family members are differentially regulated by c-erbB signaling in head and neck squamous carcinoma cells. Clin. Exp. Metastasis 18, 155–161 (2000).
Ciardiello, F. et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 7, 1459–1465 (2001).
Rak, J. et al. Ras regulation of vascular endothelial growth factor and angiogenesis. Methods Enzymol. 333, 267–283 (2001).
Ang, Y. et al. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small cell lung cancer. J. Clin. Oncol. 20, 900–910 (2002).
Giatromanolaki, A. et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br. J. Cancer 85, 881–890 (2001).
Sunwoo, J. B. et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κB, cell survival, tumour growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 7, 1419–1429 (2001).
Okada, A. et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast and head and neck carcinoma. Proc. Natl Acad. Sci. USA 92, 2730–2734 (1995).
Dewhrist, M. W. et al. Morphologic and hemodynamic comparison of tumour and healing normal tissue microvasculature. Int. J. Radiat. Oncol. Biol. Phys. 17, 91–99 (1989).
Lal, B. K., Varma, S., Pappas, P. J., Hobson, R. W. & Duran, W. N. VEGF increases permeability of the endothelial cell by activation of PKB/Akt, endothelial cell nitric-oxide synthase, and MAP kinase pathways. Microvasc. Res. 62, 252–262 (2001).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumours in vivo: simultaneous high-resolution measurements reveal a lack of correlation. Nature Med. 3, 177–182 (1997).An extremely accurate description of the modifications in the 'tumour microenviroment'. It will stimulate interest in reading and searching further information on this topic that could apply to diagnostics and therapeutics in oncology.
Graeber, T. G. et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379, 88–91 (1996).
Li, W. W. Tumour angiogenesis: molecular pathology, therapeutic targeting, and imaging. Acad. Radiol. 7, 800–811 (2000).A good overview of diagnostic opportunities in the field of angiogenesis.
Stohrer, M., Boucher, Y., Stangassinger, M. & Jain, R. K. Oncotic pressure in solid tumours is elevated. Cancer Res. 60, 4251–4255 (2000).
Hansen-Algenstaedt, N. et al. Tumour oxygenation in hormone-dependent tumours during vascular endothelial growth factor receptor-2 blockade, hormone ablation, and chemotherapy. Cancer Res. 60, 4556–4560 (2000).This article provides an opportunity to explore the close correlation between angiogenesis and hypoxia and their modifications during therapeutic intervention.
Abramovitch, R., Dafni, H., Smouha, E., Benjamin, L. E. & Neeman, M. In vivo prediction of vascular susceptibility to endothelial growth factor withdrawal: magnetic resonance imaging of C6 rat glioma in nude mice. Cancer Res. 59, 5012–5016 (1999).
Pham, C. D. et al. Magnetic resonance imaging detects suppression of tumour vascular permeability after administration of antibody to vascular endothelial growth factor. Cancer Invest. 16, 225–230 (1998).
Taylor, N. J. et al. BOLD MRI of human tumour oxygenation during carbogen breathing. J. Magn. Reson. Imaging 14, 156–163 (2001).
Van Dijke, C. F. et al. Mammary carcinoma model: correlation of macromolecular contrast-enhanced MR imaging characterizations of tumour microvasculature and histologic capillary density. Radiology 198, 813–818 (1996).A description of the potential for contrast-enhanced MRI in the detailed description of the microvasculature.
Port, R. E. et al. Multicompartment analysis of gadolinium chelate kinetics: blood–tissue exchange in mammary tumours as monitored by dynamic MR imaging. J. Magn. Reson. Imaging 10, 233–241 (1999).
Tofts, P. S. et al. Quantitative analysis of dynamic Gd–DTPA enhancement in breast tumours using a permeability model. Magn. Reson. Med. 33, 564–568 (1995).
Brasch, R. C et al. In vivo monitoring of tumour angiogenesis with MR imaging. Acad. Radiol. 7, 812–823 (2000).
Jacquemier, J. et al. Angiogenesis as a prognostic marker in breast carcinoma with conventional adjuvant chemotherapy: a multiparametric and immunohistochemical analysis. J. Pathol. 184, 130–135 (1998).
Linderholm, B. et al. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J. Clin. Oncol. 16, 3121–3128 (1998).
Fuss, M. et al. Tumour angiogenesis of low-grade astrocytomas measured by dynamic susceptibility contrast-enhanced MRI (DSC–MRI) is predictive of local tumour control after radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 51, 478–482 (2001).
Padhani, A. R. et al. Effects of androgen deprivation on prostatic morphology and vascular permeability evaluated with MR imaging. Radiology 218, 365–374 (2001).
Brasch, R. et al. Assessing tumour angiogenesis using macromolecular MR imaging contrast media. J. Magn. Reson. Imaging 7, 68–74 (1997).
Ferrara, K. W. et al. Evaluation of tumour angiogenesis with US: imaging, Doppler, and contrast agents. Acad. Radiol. 7, 824–839 (2000).
Fleischer, A. C. Sonographic depiction of tumour vascularity and flow: from in vivo models to clinical applications. J. Ultrasound Med. 11, 377–385 (1992).
Ismail, M., Peterson, R. O., Alexander, A. A., Newschaffer, C. & Gomella, L. G. Color Doppler imaging in predicting the biologic behavior of prostate cancer: correlation with disease-free survival. Urology 50, 906–912 (1997).
Lee, W. J. et al. Breast cancer vascularity: color Doppler sonography and histopathology study. Breast Cancer Res. Treat. 37, 291–298 (1996).
Donnelly, E. F., Geng, L., Wojcicki, W. E., Fleischer, A. C. & Hallahan, D. E. Quantified power Doppler US of tumour blood flow correlates with microscopic quantification of tumour blood vessels. Radiology 219, 166–170 (2001).
Rooks, V., Beecken, W.-D., Iordanescu, I. & Taylor, G. A. Sonographic evaluation of orthotopic bladder tumours in mice treated with TNP-470, an angiogenic inhibitor. Acad. Radiol. 8, 121–127 (2001).
Blankenberg, F. G. et al. Role of radionuclide imaging in trials of antiangiogenic therapy. Acad. Radiol. 7, 851–867 (2000).
Haubner, R. et al. Glycosylated RGD-containing peptides: tracer for tumour targeting and angiogenesis imaging with improved biokinetics. J. Nucl. Med. 42, 326–336 (2001).
Miles, K. A. Tumour angiogenesis and its relation to contrast enhancement on computerised tomography: a review. Eur. J. Radiol. 30, 198–205 (1999).
Blomley, M. J. K. & Dawson, P. Bolus dynamics: theoretical and experimental aspects. Br. J. Radiol. 70, 351–359 (1997).
Cenic, A., Nabavi, D. G., Craen, R. A., Gelb, A. W. & Lee, T. Y. A CT method to measure hemodynamics in brain tumours: validation and application to cerebral blood flow maps. Am. J. Neuroradiol. 21, 462–470 (2000).
Shaheen, R. M. et al. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumour and endothelial cell apoptosis. Cancer Res. 59, 5412–5416 (1999).
Solorzano, C. C. et al. In vivo intracellular signaling as a marker of antiangiogenic activity. Cancer Res. 61, 7048–7051 (2001).
Monestiroli, S. et al. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 61, 4341–4344 (2001).
Poon, R. T.-P., Fan, S.-T. & Wang, J. Clinical implications of circulating angiogenic factors in cancer patients. J. Clin. Oncol. 19, 1207–1225 (2001).A comprehensive review of the role of circulating factors (mainly VEGF and bFGF) as prognostic and predictive markers in solid malignancies.
Millauer, B. et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72, 835–846 (1993).
Millauer B et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumour types in vivo. Cancer Res. 56, 1615–1620 (1996).
Rosen, L. S. et al. Phase I dose-escalating trial of SU5416, a novel angiogenesis inhibitor in patients with advanced malignancies. Proc. Am. Soc. Clin. Oncol. 18, 618 (1999).
Gordon, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 19, 843–850 (2001).
Margolin, K. et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J. Clin. Oncol. 19, 851–856 (2001).
Rowinsky, E. K. et al. Phase I pharmacologic study of the specific metalloproteinase inhibitor BAY 12-9566 on a protacted oral daily dosing schedule in patients with solid malignancies. J. Clin. Oncol. 18, 178–186 (2000).
Gutheil, J. C. et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin ανβ3 . Clin. Cancer Res. 6, 3056–3061 (2000).
Boehle, A. S. et al. Human endostatin inhibits growth of human non-small cell lung cancer in a murine xenotransplant model. Int. J. Cancer 94, 420–428 (2001).
Fogler, W. E. et al. Recombinant human endostatin demonstrates consistent and predictable pharmacokinetics following intravenous bolus administration to cancer patients. Proc. Am. Soc. Clin. Oncol. 20, 274 (2001).
Eder, J. P. et al. A Phase I pharmacokinetic and pharmacodynamic trial of recombinant human endostatin. Proc. Am. Soc. Clin. Oncol. 20, 275 (2001).
Thomas, J. P. et al. A Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin. Proc. Am. Soc. Clin. Oncol. 20, 276 (2001).
Herbst, R. S. et al. Phase I clinical trial of recombinant human endostatin (rHE) in patients (Pts) with solid tumours: pharmacokinetic (PK), safety and efficacy analysis using surrogate endpoints of tissue and radiologic response. Proc. Am. Soc. Clin. Oncol. 20, 9 (2001).
Davis, D. W. et al. Quantitative analysis of tumour vascular targeting: effects of endostatin in a Phase I clinical trial. J. Clin. Cancer Res. 7 (Suppl.), 3711 (2001).
Soker, S. et al. Circulating endothelial progenitor cells in cancer diagnosis and therapy. J. Clin. Cancer Res. 7 (Suppl.), 3665 (2001).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumours. J. Natl Cancer Inst. 92, 205–216 (2000).A classic paper that every clinical investigator in cancer research should be familiar with.
Kopec, J. A., Abramowicz, M. & Esdail, J. M. Randomized discontinuation trials: utility and efficiency. J. Clin. Epidemiol. 46, 959–971 (1993).
Thall, P. F., Simon, R. M. & Estey, E. H. Bayesian sequential monitoring designs for single-arm trials with multiple outcomes. Stat. Med. 14, 357–379 (1995).An introduction to the complex topic of novel Bayesian monitoring methods applied to clinical-trial designs in oncology.
Ratain, M. J. et al. Clinical trial designs for cytotoxic agents J. Clin. Oncol. 19, 3154–3155 (2000).
Rosner, G. L., Stadley, W. & Ratain, M. J. The randomized discontinuation design: application to cytotoxic antineoplastic agents. J. Clin. Oncol. (in the press).
Kohn, E. C. et al. Structure–function analysis and growth inhibition by carboxyamido-triazole, CAI. Cancer Res. 54, 935–942 (1994).
Bonomi, P. Matrix metalloproteinases and matrix metalloproteinase inhibitors in lung cancer. Semin. Oncol. 29, 78–86 (2002).
Coussens, L. M., Fingleton, B. & Matrisian, L. M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2388–2392 (2002).
Khokha, R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of metalloproteinases-1. J. Natl Cancer Inst. 86, 299–304 (1994).
Gehan, E. A. & Freireich, E. J. Non-randomized controls in cancer clinical trials. N. Engl. J. Med. 290, 198–203 (1974).
Simon, R. Optimal two-stage designs for Phase II clinical trials. Control. Clin. Trials 10, 1–10 (1989).
Green, S. & Weiss, G. R. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest. New Drugs 10, 239–253 (1992).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Medscape DrugInfo
FURTHER INFORMATION
European Organisation for Research and Treatment of Cancer
The Cancer and Leukemia Group B
Glossary
- ANGIOPOIETINS
-
Angiopoietins are a novel family of proteins that specifically recognize and bind to the endothelial-cell-specific Tie2- receptor tyrosine kinase, and have been shown to be crucially involved in establishing the mature vascular network.
- MICROVASCULATURE
-
A network of vessels that have diameters of less than 100 μm, which are beyond the resolution of conventional angiography.
- MAGNETIC RESONANCE IMAGING
-
The use of radio waves in the presence of a magnetic field to extract information from certain atomic nuclei (most commonly hydrogen; for example, in water). Tissues can be differentiated by differences in their water densities. Tumours can be traced, as tumour tissue has a different water density from surrounding healthy tissue.
- CONTRAST AGENTS
-
Contrast agents enhance the differences between normal and abnormal tissue in imaging studies.
- ULTRASONOGRAPHY
-
The use of sound waves above the audible frequency to detect and characterize tumours. Echoes that are reflected off normal and abnormal tissues are captured by a computer to create two-dimensional images.
- SINGLE-PHOTON-EMISSION COMPUTED TOMOGRAPHY
-
The detection and quantification of γ-emitting radionuclides, such as 99mTc, 111In, 123I or 125I.
- POSITRON-EMISSION TOMOGRAPHY
-
An imaging technique that is used to detect decaying nuclides, such as 15O, 13N, 11C, 18F, 124I and 94mTc.
- COMPUTED TOMOGRAPHY
-
A technique that exploits the differences in absorption of X-rays by different tissues to give high-contrast images of anatomical structures. Computed tomography has relatively poor soft-tissue contrast, so iodinated contrast agents, which perfuse different tissue types at different rates, are commonly used to delineate tumours.
- GRADE III OR IV TOXICITIES
-
For each adverse event (AE) that is associated with a specific treatment, grades are assigned and defined using a scale from 0 to V. Grade III, severe and undesirable AE; grade IV, life threatening or disabling AE.
Rights and permissions
About this article
Cite this article
Cristofanilli, M., Charnsangavej, C. & Hortobagyi, G. Angiogenesis modulation in cancer research: novel clinical approaches. Nat Rev Drug Discov 1, 415–426 (2002). https://doi.org/10.1038/nrd819
Issue date:
DOI: https://doi.org/10.1038/nrd819
This article is cited by
-
Angiogenesis imaging study using interim [18F] RGD-K5 PET/CT in patients with lymphoma undergoing chemotherapy: preliminary evidence
EJNMMI Research (2021)
-
Light-controlled calcium signalling in prostate cancer and benign prostatic hyperplasia
Future Journal of Pharmaceutical Sciences (2020)
-
Circulating VEGF and eNOS variations as predictors of outcome in metastatic colorectal cancer patients receiving bevacizumab
Scientific Reports (2017)
-
Contribution of in vitro comparison of colorectal carcinoma cells from primary and metastatic lesions to elucidation of mechanisms of tumor progression and response to anticancer therapy
Tumor Biology (2016)
-
Building better monoclonal antibody-based therapeutics
Nature Reviews Cancer (2015)