Key Points
-
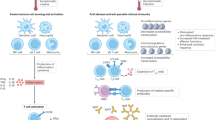
Innate immune responses have been shown to contribute to the control of malaria infections in mice and there is indirect evidence that they also contribute to the control of infection in humans.
-
There is conflicting evidence on the effect of malaria infection on dendritic cells (DCs), with conventional activation of DCs reported in some model systems and modulation of DC function towards an immunoregulatory phenotype reported in other systems.
-
Natural killer T (NKT) cells have a role in immunity to liver-stage parasites in mice, but there is little evidence so far for a role for these cells in the response to human malaria.
-
γδ T cells are activated in malaria infections in both mice and humans, and contribute to parasite clearance, but this role does not seem to be essential; some studies indicate that they might contribute to immune-mediated pathology.
-
NK cells are frequently the earliest source of interferon-γ during a blood-stage malaria infection and have an essential role in controlling acute parasitaemia in mice. Heterogeneity of the NK-cell response in humans indicates that their activation might be influenced by polymorphic NK-cell receptors.
-
Polymorphisms have been described in many genes encoding components of the innate immune response and there is evidence that such polymorphisms might affect the outcome of infection in humans.
-
Although a robust pro-inflammatory cytokine response emanating from cells of the innate immune system might be important for controlling acute infection, the potential for these responses to contribute to immune-mediated pathology needs to be investigated.
-
Innate immune responses influence the nature and magnitude of the adaptive immune response to malaria. Activators of the innate response might therefore be incorporated into malaria vaccines to provide adjuvant activity.
Abstract
Malaria is a major cause of disease and death in tropical countries. A safe and effective vaccine is essential to achieve significant and sustained reductions in malaria-related morbidity and mortality. Driven by this need, research on the immunology of malaria has tended to focus on adaptive immunity. The potential for innate immune mechanisms to provide rapid protection against malaria has been largely neglected. On the basis of data from animal models, and clinical and epidemiological studies, this review considers the potential for innate immune mechanisms directed against Plasmodium parasites both to contribute to protection from malaria and to modulate adaptive immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Malaria: Parasite Biology, Pathogenesis and Protection. (ed. Sherman, I. W.) (ASM Press, Washington DC, 1998).
Good, M. F. & Doolan, D. L. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11, 412–419 (1999).
Good, M. F. Towards a blood-stage vaccine for malaria: are we following all the leads? Nature Rev. Immunol. 1, 117–125 (2001).
Schofield, L. et al. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418, 785–789 (2002). This paper shows a causal association between malarial glycosylphosphatidylinositol (GPI) and severe pathology in mice.
Franks, S., et al. Frequent and persistent asymptomatic Plasmodium falciparum infections in African infants characterised by multilocus genotyping. J. Infect. Dis. 183, 796–804 (2001).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002).
Kwiatkowski, D. et al. The malarial fever response — pathogenesis, polymorphism and prospects for intervention. Ann. Trop. Med. Parasitol. 91, 533–542 (1997).
Hunt, N. & Grau, G. E. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24, 491–499 (2003).
Langhorne, J., Quin, S. J. & Sanni L. A. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem. Immunol. 80, 204–228 (2002).
Fortin, A., Stevenson, M. M. & Gros, P. Susceptibility to malaria as a complex trait: huge pressure from a tiny creature. Hum. Mol. Genetics 11, 2469–2478 (2002).
de Souza, J. B. & Riley, E. M. Cerebral malaria: the contribution of studies in animals to our understanding of immunopathogenesis. Microbes Infect. 4, 291–300 (2002).
Shear, H. L., Marino, M. W., Wanidworanun, C. J., Berman, W. & Nagel, R. L. Correlation of increased expression of intercellular adhesion molecule-1, but not high levels of tumor necrosis factor-α, with lethality of Plasmodium yoelii 17XL, a rodent model of cerebral malaria. Am. J. Trop. Med. Hyg. 59, 852–858 (1998).
McQueen, K. & Parham, P. Variable receptors controlling activation and inhibition of NK cells. Curr. Opin. Immunol. 14, 615–621 (2002).
Colucci, F., Di Santo, J. P. & Leibson, P. J. Natural killer cell activation in mice and men: different triggers for similar weapons? Nature Immunol. 3, 807–813 (2002).
Shortman, K. & Liu, Y. -J. Mouse and human dendritic cell subtypes. Nature Rev. Immunol. 2, 151–161 (2002).
Su, Z. & Stevenson, M. M. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria. J. Immunol. 168, 1348–1355 (2002). This is the first paper to show the importance of a T helper 1 (T H 1)-cell response for protective immunity during both the acute and chronic phases of blood-stage infection with Plasmodium chabaudi chabaudi AS, and the role of interleukin-12 (IL-12) in inducing cell-mediated and antibody-mediated immunity to malaria.
Stevenson, M. M., Su, Z., Sam, H. & Mohan, K. Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes Infect. 3, 49–59 (2001).
van der Heyde, H. C., Pepper, B., Batchelder, J., Cigel, F. & Weidanz, W. P. The time course of selected malarial infections in cytokine-deficient mice. Exp. Parasitol. 85, 206–213 (1997). References 18 and 20 provide evidence for the essential role of interferon-γ (IFN-γ) in protective immunity to blood-stage infection with P. chabaudi species and Plasmodium yoelii.
Su, Z. & Stevenson, M. M. Central role of endogenous γ-interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68, 4399–4406 (2000).
Meding, S. J. & Langhorne, J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur. J. Immunol. 21, 1433–1438 (1991).
Choudhury, H., Sheikh, N., Bancroft, G., Katz, D. & De Souza, J. Early nonspecific immune responses and immunity to blood-stage nonlethal Plasmodium yoelii malaria. Infect. Immun. 68, 6127–6132 (2000).
Mohan, K., Moulin, P. & Stevenson, M. M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J. Immunol. 159, 4990–4998 (1997).
de Souza, J. B., Williamson, K. H., Otani, T. & Playfair, J. H. L. Early γ-interferon responses in lethal and nonlethal murine blood stage malaria. Infect. Immun. 65, 1593–1598 (1997). References 21–23 show that the production of IFN-γ by natural killer (NK) cells and possibly γδ T cells early in infection is important in activating the appropriate CD4+ T H -cell subset during blood-stage malaria in mice.
Molineaux, L., Trauble, M., Collins, W., Jeffery, G. & Dietz, K. Malaria therapy reinoculation data suggest individual variation of an innate immune response and independent acquisition of antiparasitic and antitoxic immunities. Trans. R. Soc. Trop. Med. Hyg. 96, 205–209 (2002).
Luty, A., Kun, J. & Kremsner, P. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J. Infect. Dis. 178, 1221–1224 (1998).
Burgner, D., et al. Nucleotide and haplotypic diversity of the NOS2A promoter region and its relationship to cerebral malaria. Hum. Genetics 112, 379–386 (2003).
Kun, J. et al. Nitric oxide synthase 2Lambarene (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 184, 330–336 (2001).
Hobbs, M. et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360, 1468–1475 (2002).
Hill, A. V. S. et al. Common West African HLA antigens are associated with protection from severe malaria. Nature 352, 595–600 (1991).
Koch, O. et al. IFNGR1 gene promoter polymorphisms and susceptibility to cerebral malaria. J. Infect. Dis. 185, 1684–1687 (2002).
Aucan, C. et al. Interferon-α receptor-1 (IFNAR1) variants are associated with protection against cerebral malaria in The Gambia. Genes Immun. 4, 275–282 (2003).
Omi, K. et al. CD36 polymorphism is associated with protection from cerebral malaria. Am. J. Hum. Genet. 72, 364–374 (2003).
Aitman, T. et al. Malaria susceptibility and CD36 mutation. Nature 405, 1015–1016 (2000).
Pain, A. et al. A non-sense mutation in CD36 gene is associated with protection from severe malaria. Lancet 357, 1502–1503 (2001).
Artavanis-Tsakonas, K. et al. Activation of a subset of human natural killer cells upon contact with Plasmodium falciparum-infected erythrocytes. J. Immunol. 171, 5396–5405 (2003).
Sabeti, P. et al. CD40L association with protection from severe malaria. Genes Immun. 3, 286–291 (2002).
McGuire, W., Hill A. V. S., Allsopp, C. E. M., Greenwood, B. M. & Kwiatkowski, D. Variation in the TNFα promoter region associated with susceptibility to cerebral malaria. Nature 371, 508–511 (1994). This study was the first to show a relationship between a cytokine gene polymorphism and risk of severe malaria, indicating the need to regulate inflammatory cytokine levels to prevent severe pathology.
Knight, J. C. et al. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nature Genet. 22, 145–150 (1999).
Luoni, G. et al. Antimalarial antibody levels and IL-4 polymorphism in the Fulani of West Africa. Genes Immun. 2, 411–414 (2001).
Morahan, G., et al. A promoter polymorphism in the gene encoding interleukin-12 p40 (IL12B) is associated with mortality from cerebral malaria and with reduced nitric oxide production. Genes Immun. 3, 414–418 (2002).
Kwiatkowski, D. Genetic susceptibility to malaria getting complex. Curr. Opin. Genet. Dev. 10, 320–324 (2000).
Hermsen, C. et al. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin. Exp. Immunol. 132, 467–472 (2003).
Scragg, I., Hensmann, M., Bate, C. & Kwiatkowski, D. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur. J. Immunol. 29, 2636–2644 (1999).
Bruce, M. & Day, K. P. Cross-species regulation of Plasmodium parasitemia in semi-immune children from Papua New Guinea. Trends Parasitol. 19, 271–277 (2003). This article describes how innate immune responses can control, but not eliminate, malaria infections and that innate effector mechanisms are equally effective against different species and strains of malaria.
Kwiatkowski, D. & Nowak, M. Periodic and chaotic host-parasite interactions in human malaria. Proc. Natl Acad. Sci. USA 88, 5111–5113 (1991).
Richie, T. L. Interactions between malaria parasites infecting the same vertebrate hosts. Parasitology 96, 607–639 (1988).
Maitland, K., Williams, T. N. & Newbold, C. Plasmodium vivax and P. falciparum: biological interactions and the possibility of cross-species immunity. Parasitol. Today 13, 227–231 (1997).
Ewald, P. W. Evolution of Infectious Disease (Oxford University Press, New York, 1994).
Scharton-Kersten, T., Afonso, L., Wysocka, M., Trinchieri, G. & Scott, P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154, 5320–5330 (1995).
Biron, C. A. Activation and function of natural killer responses during viral infections. Curr. Opin. Immunol. 9, 24–34 (1997).
Unanue, E. R. Inter-relationship among macrophages, natural killer cells, and neutrophils in early stage of Listeria resistance. Curr. Opin. Immunol. 9, 35–43 (1997).
Scharton-Kersten, T. & Sher, A. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 9, 44–51 (1997).
Quin, S. J. et al. Low CD4+ T cell responses to the C-terminal region of the malaria merozoite surface protein-1 may be attributed to processing within distinct MHC class II pathways. Eur. J. Immunol. 31, 72–81 (2001).
Luyendyk, J., Olivas, O. R., Ginger, L. A. & Avery, A. C. Antigen-presenting cell function during Plasmodium yoelii infection. Infect. Immun. 70, 2941–2949 (2002).
McGregor, I. A. & Barr, M. Antibody response to tetanus toxoid inoculation in malarious and non-malarious Gambian children. Trans. R. Soc. Med. Hyg. 56, 364–367 (1962).
Greenwood, B. M., Bradley-Moore, A. M., Palit, A. & Bryceson, A. D. M. Immunosuppression in children with malaria. Lancet 1, 169–172 (1972).
Serghides, I., Smith, T. G., Patel, S. N. & Kain, K. C. CD36 and malaria: friends or foes? Trends Parasitol. 19, 461–469 (2003).
Guermonprez, P., Valladeau, J., Zitvogel, L., Théry, C. & Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002).
Sher, A., Pearce, E. & Kaye, P. Shaping the immune response to parasites: role of dendritic cells. Curr. Opin. Immunol. 15, 421–429 (2003).
Robinson, B., Welch, T. & Smith, J. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 47, 1265–1278 (2003).
Urban, B. C. et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400, 73–77 (1999). This was the first report of the ability of Plasmodium parasites to inhibit dendritic-cell (DC) maturation and antigen-presenting function in vitro.
Eda, S. & I. Sherman . Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell Physiol. Biochem. 12, 373–384 (2002).
Tachado, S. D. et al. Glycophosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J. Immunol. 156, 1897–1907 (1996).
Vijaykumar, M., Naik, R. & Gowda, D. Plasmodium falciparum glycosylphosphatidylinositol-induced TNF-α secretion by macrophages is mediated without membrane insertion or endocytosis. J. Biol. Chem. 276, 6909–6912 (2001).
Adachi, K. et al. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 167, 5928–5934 (2001).
Behr, C. et al. Plasmodium falciparum stimuli for human γδ T cells are related to the phophorylated antigens of mycobacteria. Infect. Immun. 64, 2892–2896 (1996).
Pichyangkul, S. et al. Activation of γδ T cells in malaria: interaction of cytokines and a schizont-associated Plasmodium falciparum antigen. J. Infect. Dis. 176, 233–241 (1997).
Orago, A. & Facer, C. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin. Exp. Immunol. 86, 22–29 (1991).
Schofield, L. et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283, 225–229 (1999).
Lee, S. Gonzalez-Aseguinolaza, G. & Nussenzweig, M. Disseminated candidiasis and hepatic malarial infection in mannose-binding-lectin-A-deficient mice. Mol. Cell Biol. 22, 8199–8203 (2002).
Klabunde, J. et al. Recognition of Plasmodium falciparum proteins by mannan-binding lectin, a component of the human innate immune system. Parasitol. Res. 88, 113–117 (2002).
Claudianos, C. et al. A malaria scavenger receptor-like protein essential for parasite development. Mol. Microbiol. 45, 1473–1484 (2002).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Campos, M. A. et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167, 416–423 (2001).
Gramzinski, R. et al. Interleukin-12- and γ-interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69, 1643–1649 (2001).
Su, Z, Tam, M. -F., Jankovic, D. & Stevenson, M. M. Vaccination with novel immunostimulatory adjuvants against blood-stage malaria in mice. Infect. Immun. 71, 5178–5187 (2003).
Near, K. A., Stowers, A. W., Jankovic, D. & Kaslow, D. C. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect. Immun. 70, 692–701 (2002). The first example of a pathogen-associated activator of the innate immune response that enhances vaccine-induced immunity against Plasmodium.
Ocana-Morgner, C., Mota, M. & Rodriguez, A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197, 143–151 (2003).
Perry, J. A., Rush, A., Wilson, R. J., Olver, C. S. & Avery, A. C. Dendritic cells from malaria-infected mice are fully functional APC. J. Immunol. 172, 475–482 (2004).
Seixas, E., Cross, C., Quin, S. & Langhorne, J. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur. J. Immunol. 31, 2970–2978 (2001).
Coban, C., Ishii, K., Sullivan, D. & Kumar, N. Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect. Immun. 70, 3939–3943 (2002).
Urban, B. et al. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood 98, 2859–2861 (2001).
Singh, R. P. et al. The role of IL-18 in blood-stage immunity against murine malaria Plasmoidum yoelii 265 and Plasmodium berghei ANKA. J. Immunol. 168, 4674–4681 (2002).
Luty, A. et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68, 3905–3915 (2000).
Malaguarnera, L. et al. Increased levels of interleukin-12 in Plasmodium falciparum malaria: correlation with the severity of disease. Parasite Immunol. 24, 387–389 (2002).
Perkins, D., Weinberg, J. & Kremsner, P. Reduced interleukin-12 and transforming growth factor-β1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182, 988–992 (2000).
Malaguarnera, L., Pignatelli, S., Musumeci, M., Simpore, J. & Musumeci, S. Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol. 24, 489–492 (2002).
Dodoo, D. et al. Absolute levels and ratios of pro-inflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to P. falciparum malaria. J. Infect. Dis. 185, 971–979 (2002).
Schmieg, J., Gonzalez-Asequinolaza, G. & Tsuji, M. The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect. 5, 499–506 (2003).
Hansen, D., Siomos, M., Buckingham, L., Scalzo, A. & Schofield, L. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18, 391–402 (2003).
Procopio, D. O. et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169, 3926–3933 (2002).
Langhorne, J., Morris-Jones, S., Casabo, L. G. & Goodier, M. The response of γδ T cells in malaria infections: a hypothesis. Res. Immunol. 145, 429–436 (1994).
Hviid, L. et al. Perturbation and proinflammatory type activation of V δ1+ γδT cells in African children with Plasmodium falciparum malaria. Infect. Immun. 69, 3190–3196 (2001).
Hensmann, M. & Kwiatkowski, D. Cellular basis of early cytokine response to Plasmodium falciparum. Infect. Immun. 69, 2364–2371 (2001).
Elloso, M. M., van der Heyde, H. C., Vande Waa, J. A., Manning, D. D. & Weidanz, W. P. Inhibition of Plasmodium falciparum in vitro by human γδ T cells. J. Immunol. 153, 1187–1194 (1994).
Constant, P. et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264, 267–270 (1994).
Tanaka, Y. et al. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 375, 155–158 (1995).
Elloso, M. M., van der Heyde, H. C., Troutt, A., Manning, D. D. & Weidanz, W. P. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J. Immunol. 157, 2096–2102 (1996).
Waterfall, M., Black, A. & Riley, E. γδ+ T cells preferentially respond to live rather than killed malaria parasites. Infect. Immun. 66, 2393–2398 (1998).
Morris-Jones, S., Goodier, M. & Langhorne, J. The response of γδ T cells to Plasmodium falciparum is dependent on activated CD4+ T cells and the recognition of MHC class I molecules. Immunol. 89, 405–412 (1996).
van der Heyde, H. C. et al. γδ T cells function in cell-mediated immunity to acute blood-stage Plasmodium chabaudi adami malaria. J. Immunol. 154, 3985–3990 (1995).
Seixas, E. & Langhorne, J. γδ T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J. Immunol. 162, 2837–2841 (1999).
Langhorne, J., Mombaerts, P. & Tonegawa, S. αβ and γδ T cells in the immune response to the erythrocytic stages of malaria in mice. Int. Immunol. 7, 1005–1011 (1995).
Weidanz, W. et al. Plasticity of immune responses suppressing parasitemia during acute Plasmodium chabaudi malaria. J. Immunol. 162, 7383–7388 (1999).
Yanez, D., Batchelder, J., van der Heyde, H., Manning, D. & Weidanz, W. γδ T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect. Immun. 67, 446–448 (1999).
Tsuji, M. et al. Phenotypic and functional properties of murine γδ T cell clones derived from malaria immunized αβ T cell-deficient mice. Int. Immunol. 8, 359–366 (1996).
Kopacz, J. & Kumar, N. Murine γδ T lymphocytes elicited during Plasmodium yoelii infection respond to Plasmodium heat shock proteins. Infect. Immun. 67, 57–63 (1999).
Moretta, A., Bottino, C., Mingari, M., Biassoni, R. & Moretta, L. What is a natural killer cell? Nature Immunol. 3, 6–8 (2002).
Ojo-Amaize, E., Vilcek, J., Cochrane, A. & Nussenzweig, R. Plasmodium berghei sporozoites are mitogenic for murine T cells, induce interferon, and activate natural killer cells. J. Immunol. 133, 1005–1009 (1984).
Pasquetto, V., Guidotti, L., Kakimi, K., Tsuji, M. & Chisari, F. Host-virus interactions during malaria infections in hepatitis B virus transgenic mice. J. Exp. Med. 192, 529–536 (2000).
Doolan, D. L. & Hoffman, S. L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163, 884–892 (1999).
Artavanis-Tsakonas, K. & Riley, E. M. Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169, 2956–2963 (2002). This paper provides evidence for direct, contact-dependent recognition of Plasmodium falciparum -infected erythrocytes by NK cells, implying that infected erythrocytes express ligands for NK-cell receptors.
Theander, T. G. et al. Enhancement of human natural cytotoxicity by Plasmodium falciparum antigen activated lymphocytes. Acta Trop. 44, 415–422 (1987).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Wattavidanage, J. et al. TNF-α*2 marks high risk of severe disease during Plasmodium falciparum malaria and other infections in Sri Lankans. Clin. Exp. Immunol. 115, 350–355 (1999).
Aidoo, M. et al. Tumor necrosis factor-α promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet. Epidemiol. 21, 201–211 (2001).
Ubalee, R. et al. Strong association of a tumor necrosis factor-α promoter allele with cerebral malaria in Myanmar. Tissue Antigens 58, 407–410 (2001).
Uhrberg, M. et al. Human diversity in killer cell inhibitory receptor genes. Immunity 7, 753–763 (1997).
Rajalingam, R., Gardiner, C., Canavez, F., Vilches, C. & Parham, P. Identification of seventeen novel KIR variants: fourteen of them from two non-Caucasian donors. Tissue Antigens 57, 22–31 (2001).
Akalin, E. & Murphy, B. Gene polymorphisms and transplantation. Curr. Opin. Immunol. 13, 572–576 (2001).
Arbour, N. et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nature Genet. 25, 187–191 (2000).
Summerfield, J. et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet 345, 886–889 (1995).
Riley, E. M. Is T cell priming required for initiation of pathology in malaria infections? Immunol. Today 20, 228–233 (1999).
Engwerda, C. et al. Locally upregulated lymphotoxin α, not systemic tumor necrosis factor α, is the principle mediator of murine cerebral malaria. J. Exp. Med. 195, 1371–1377 (2002).
Yoshimoto, T. et al. Pathogenic role of IL-12 in blood-stage murine malaria lethal strain Plasmodium berghei NK65 infection. J. Immunol 160, 5500–5505 (1998).
Knight, J. C., Keating, B. J., Rockett, K. A. & Kwiatkowski, D. P. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nature Genet. 33, 469–475 (2003).
Omer, F. M. & Riley, E. M. TGF-β production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 188, 39–48 (1998). This was the first study to show a causal relationship between the balance of pro-inflammatory and antiinflammatory cytokines and the outcome of malaria infections.
Omer, F., de Souza, J. & Riley, E. Differential induction of TGF-β regulates pro-inflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J. Immunol. 171, 5430–5436 (2003).
Li, C., Sanni, L. A., Omer, F. M., Riley, E. M. & Langhorne, J. Pathology and mortality of Plasmodium chabaudi chabaudi infection in IL-10-deficient mice is ameliorated by anti-TNF-α and exacerbated by anti-TGF-β antibodies. Infect. Immun. 71, 4850–4856 (2003).
Kurtzhals, J. A. L. et al. Low concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351, 1768–1772 (1998).
Tsutsui, N. & Kamiyama, T. ransforming growth factor β-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect. Immun. 67, 2306–2311 (1999).
Omer, F. M., de Souza, J. B., Corran, P. H., Sultan, A. A. & Riley, E. M. Activation of transforming growth factor-β by malaria parasite-derived metalloproteases and a thrombospondin-like molecule. J. Exp. Med. 198, 1817–1827 (2003).
Ortaldo, J. R. et al. Mechanistic studies of transforming growth factor-β inhibition of IL-2-dependent activation of CD3− large granular lymphocyte functions. Regulation of IL-2Rβ (p75) signal transduction. J. Immunol. 146, 3791–3798 (1991).
D'Andrea, A., Aste-Amezaga, M., Valiante, N., Ma, X., Kubin, M. & Trinchieri, G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178, 1041–1048 (1993).
Roura-Mir, C. & Moody, B. Sorting out self and microbial lipid antigens for CD1. Microbes Immun. 5, 1137–1148 (2003).
Sköld, M. & Behar, S. M. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71, 5447–5455 (2003).
Hansen, D. S. et al. CD1d-restricted NKT cells contribute to malaria splenomegaly and enhance parasite-specific antibody responses. Eur. J. Immunol. 33, 2588–2598 (2003).
Weiss, W. R. et al. A plasmid encoding murine granulocyte–macrophage colony stimulating factor increases protection conferred by a malaria DNA vaccine. J. Immunol. 161, 2325–2332 (1998).
Acknowledgements
We gratefully acknowledge the assistance of M. Tam in the preparation of this manuscript. E.M.R. would like to acknowledge the contributions of K. Artavanis-Tsakonas, M. Walther, D. Korbel, D. Davis, K. McQueen and P. Parham to studies on innate immunity in her laboratory. The work is supported by the UK Medical Research Council, The Wellcome Trust, Boehringer Ingelheim Funds, The Burroughs Wellcome Fund and the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- γδ T CELLS
-
Although γδ T-cell receptors are potentially diverse, circulating γδ T cells express a restricted set of these receptors and seem to recognize a relatively restricted set of ligands; this might reflect postnatal expansion of a small number of γδ T-cell clones by a few potent antigens, such as those expressed by mycobacteria or other widely distributed bacteria.
- SEVERE COMBINED IMMUNODEFICIENT (SCID) MICE
-
Mice with this defect in their immune system do not have B or T cells and can, therefore, not mount adaptive immune responses.
- NUDE MICE
-
A mutation in mice that causes both hairlessness and defective formation of the thymus, which results in a lack of mature T cells.
- CYTOPHILIC ANTIBODY
-
Opsonizing antibody subclasses in mice (IgG2a and IgG2b) and in humans (IgG1 and IgG3), which mediate phagocytosis by macrophages.
- UNMETHYLATED CpG MOTIFS
-
Sequences in bacterial DNA recognized by the mammalian immune system, which consist of unmethylated CpG dinucleotides in certain base contexts.
- MIXED LYMPHOCYTE REACTION
-
(MLR). When peripheral-blood mononuclear cells or splenocytes from MHC-disparate donors are mixed together in the same culture, T helper cells from each donor recognize allogeneic MHC molecules on antigen-presenting cells from the other donor, and the T helper cells are induced to proliferate and release cytokines.
- NKT CELLS
-
A heterogeneous population of lymphocytes with phenotypic and functional characteristics of both classical T cells and natural killer (NK) cells. Classical mouse NKT cells express the NK1.1 cell-surface marker, are T-cell receptor (TCR) Vα14+, recognize lipid-containing antigen in the context of the non-classical MHC class I molecule CD1d and are selectively activated by the synthetic ligand α-galactosylceramide. Various unconventional T cells have also now been described that express a diverse array of TCRs and are not CD1d restricted.
Rights and permissions
About this article
Cite this article
Stevenson, M., Riley, E. Innate immunity to malaria. Nat Rev Immunol 4, 169–180 (2004). https://doi.org/10.1038/nri1311
Issue date:
DOI: https://doi.org/10.1038/nri1311
This article is cited by
-
Effect of dietary intervention on the prevalence of asymptomatic malaria among 6–18-month-old children in rural Malawi
Malaria Journal (2023)
-
Increased interferon-γ levels and risk of severe malaria: a meta-analysis
Scientific Reports (2022)
-
IFN-λ4 genetic variants influence clinical malaria episodes in a cohort of Kenyan children
Malaria Journal (2021)
-
Maternal natural killer cells at the intersection between reproduction and mucosal immunity
Mucosal Immunology (2021)
-
Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model
Cell Communication and Signaling (2020)