Key Points
-
Infections with bacterial pathogens remain a major health problem, causing more than 5 million deaths annually. The major culprits are pneumococci and meningococci in both the developing and developed world; agents of nosocomial infections, many of them being multidrug-resistant, mostly in the industrialized world; tuberculosis, which afflicts >85% of the population in developing countries and <15% in industrialized countries; and bacterial pathogens, which cause food-borne diseases that frequently have diarrhoeal sequelae (not covered in this article).
-
Vaccines are amongst the most successful measures of modern medicine — they save several million lives annually and have remarkable cost efficiency. Bacterial pathogens that are controlled successfully by vaccines include Clostridium tetani, Corynebacterium diphtheriae and Haemophilus influenzae b. Conjugate vaccines against meningococci and pneumococci are also available but need further improvements.
-
Vaccines achieve different outcomes. Most vaccines prevent disease outbreak rather than infection itself. Yet, in many cases, vaccinated individuals ultimately eradicate the pathogen. In more complex diseases, however, vaccinated individuals can only control infection if the bacterial load remains under the threshold for active disease. In such individuals, becoming immunocompromised can allow disease outbreak despite previous vaccination.
-
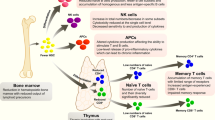
Rational vaccine design can benefit from recent insights into the immunology of host defences against infectious agents. This includes our better understanding of: how the innate immune response senses infectious agents; how it instructs the acquired immune response to develop the most appropriate defence mechanisms; the pathways through which antigens are presented to T cells; the cytokines and co-stimulatory cell-surface molecules which fine-tune T- and B-cell responses; the cytokines produced by T cells that help other immune cells to perform effector functions; and the mechanisms underlying memory for B cells and T cells. Although successful vaccines mostly rely on antibody production, the next generation of vaccines will need to exploit T cells to achieve the required efficacy.
-
Novel vaccination strategies can be roughly separated into three groups. First, vaccines based on antibody-mediated protection could lead to improved vaccines against pneumococci and meningococci as well as against nosocomial infections. Second, vaccines based on T-cell immunity will be needed against intracellular pathogens, notably Mycobacterium tuberculosis and Chlamydia trachomatis. Third, novel vaccines that prevent infection will be needed for diseases in which existing vaccines prevent disease outbreak by reducing the bacterial load under the threshold of active disease.
Abstract
In most cases, a successful vaccine must induce an immune response that is better than the response invoked by natural infection. Vaccines are still unavailable for several bacterial infections and vaccines to prevent such infections will be best developed on the basis of our increasing insights into the immune response. Knowledge of the signals that determine the best possible acquired immune response against a given pathogen — comprising a profound T- and B-cell memory response as well as long-lived plasma cells — will provide the scientific framework for the rational design of novel antibacterial vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Behring, E. Die Blut Serum Therapie zur Diphterie Behandlung des Menschen. Berliner klin. Wochenschr. 31, 827 (1894).
WHO. World Health Report 2006: working together for health. WHO, Geneva (2006).
Lowy, F. D. Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998).
Wright, G. D. The antibiotic resistome: the nexus of chemical and genetic diversity. Nature Rev. Microbiol. 5, 175–186 (2007).
Peacock, S. J. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 771–832 (Hodder Arnold, London, 2005).
Teixeira, L. M. & Facklam, R. R. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 882–902 (Hodder Arnold, London, 2005).
Robinson, D. A. et al. Re-emergence of early pandemic Staphylococcus aureus as a community-acquired meticillin-resistant clone. Lancet 365, 1256–1258 (2005).
Palleroni, N. J. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 1591–1606 (Hodder Arnold, London, 2005).
Levy, S. B. & Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Med. 10, S122–S129 (2004).
Murray, P. R., Holmes, B. & Auken, H. M. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 1474–1506 (Hodder Arnold, London, 2005).
Mims, C. et al. in Medical Microbiology 556–566 (Elsevier Mosby, Edinburgh, 2004).
Odeh, R. & Quinn, J. P. Problem pulmonary pathogens: Pseudomonas aeruginosa. Semin. Respir. Crit. Care Med. 21, 331–339 (2000).
Rappuoli, R., Miller, H. I. & Falkow, S. The intangible value of vaccination. Science 297, 937–939 (2002).
Manz, R. A., Hauser, A. E., Hiepe, F. & Radbruch, A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23, 367–386 (2005).
Casadevall, A. & Pirofski, L. A. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv. Immunol. 91, 1–44 (2006).
Verez-Bencomo, V. et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 305, 522–525 (2004). Describes large-scale synthesis of a conjugate vaccine against Haemophilus influenzae type b and protection data from clinical trials.
Posfay-Barbe, K. M. & Wald, E. R. Pneumococcal vaccines: do they prevent infection and how? Curr. Opin. Infect. Dis. 17, 177–184 (2004).
Girard, M. P., Preziosi, M. P., Aguado, M. T. & Kieny, M. P. A review of vaccine research and development: meningococcal disease. Vaccine 24, 4692–4700 (2006).
Mowat, A. M. & Garside, P. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 389–402 (Hodder Arnold/ASM Press, London/Washington D.C., 2005).
Salerno-Goncalves, R. & Sztein, M. B. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 14, 536–542 (2006).
Zanetti, M. & Franchini, G. T cell memory and protective immunity by vaccination: is more better? Trends Immunol. 27, 511–517 (2006).
Pulendran, B. & Ahmed, R. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124, 849–863 (2006).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Kalia, V., Sarkar, S., Gourley, T. S., Rouse, B. T. & Ahmed, R. Differentiation of memory B and T cells. Curr. Opin. Immunol. 18, 255–264 (2006).
Jiang, H. & Chess, L. Regulation of immune responses by T cells. N. Engl. J. Med. 354, 1166–1176 (2006).
Belkaid, Y. & Rouse, B. T. Natural regulatory T cells in infectious disease. Nature Immunol. 6, 353–360 (2005).
Mora, J. R. & von Andrian, U. H. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 27, 235–243 (2006).
Neutra, M. R. & Kozlowski, P. A. Mucosal vaccines: the promise and the challenge. Nature Rev. Immunol. 6, 148–158 (2006).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Inohara, N., Chamaillard, M., McDonald, C. & Nunez, G. NOD-LRR proteins: role in host–microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383 (2005).
Chamaillard, M., Girardin, S. E., Viala, J. & Philpott, D. J. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell. Microbiol. 5, 581–592 (2003).
Meylan, E., Tschopp, J. & Karin, M. Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 (2006).
Colonna, M., Pulendran, B. & Iwasaki, A. Dendritic cells at the host–pathogen interface. Nature Immunol. 7, 117–120 (2006).
Jackson, D. C. et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl Acad. Sci. USA 101, 15440–15445 (2004).
Unanue, E. R. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 375–388 (Hodder Arnold/ASM Press, London/Washington, D.C., 2005).
Yewdell, J. W. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 403–418 (Hodder Arnold/ASM Press, London/Washington D.C., 2005).
Kaufmann, S. H. E. in Fundamental Immunology, 5th edn (ed. Paul, W. E.) 1229–1261 (Lippincott-Raven, Philadelphia, New York, 2003).
Hayday, A. & Steele, C. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 435–448 (Hodder Arnold/ASM Press, London/Washington D.C., 2005).
Bonneville, M. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 449–470 (Hodder Arnold/ASM Press, London/Washington, D.C., 2005).
Romagnani, S. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 273–299 (Hodder Arnold/ASM Press, London/Washington, D.C., 2005).
Hsu, S.-C. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 419–434 (Hodder Arnold/ASM Press, London/Washington, D.C., 2005).
Winau, F., Hegasy, G., Kaufmann, S. H. E. & Schaible, U. E. No life without death —apoptosis as prerequisite for T cell activation. Apoptosis 10, 707–715 (2005).
Winau, F. et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24, 105–117 (2006). The authors present mechanisms underlying the processing and presentation of antigens within vesicles from cells that undergo apoptosis as a result of bacterial infection. The studies provide further evidence for the efficacy of cross-priming induced by vaccines.
Yrlid, U. & Wick, M. J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191, 613–624 (2000).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Med. 9, 1039–1046 (2003).
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Colgan, J. & Rothman, P. All in the family: IL-27 suppression of TH17 cells. Nature Immunol. 7, 899–901 (2006).
Tato, C. M., Laurence, A. & O'Shea, J. J. Helper T cell differentiation enters a new era: le roi est mort; vive le roi! J. Exp. Med. 203, 809–812 (2006).
Dong, C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature Rev. Immunol. 6, 329–333 (2006).
Weaver, C. T., Harrington, L. E., Mangan, P. R., Gavrieli, M. & Murphy, K. M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24, 677–688 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Happel, K. I. et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769 (2005). Provides the first evidence for a role of T H 17 cells in defence against the extracellular pathogen Klebsiella pneumoniae.
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 8, 369–377 (2007). Establishes a role for T H 17 cells in TB by showing that T H 17 cells can guide T H 1 cells to the infected lung.
Belkaid, Y., Piccirillo, C. A., Mendez, S., Shevach, E. M. & Sacks, D. L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420, 502–507 (2002). An interesting study describing the role of T Reg cells in chronic infection. Notably, this paper reveals not only a detrimental but also a beneficial role for T Reg cells in chronic infections.
Ndejembi, M. P., Tang, A. L. & Farber, D. L. Reshaping the past: strategies for modulating T-cell memory immune responses. Clin. Immunol. 122, 1–12 (2007).
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). Important study describing how IL-6 controls the generation of T Reg cells and of T H 17 cells stimulated by TGF-β. The presence of IL-6 favours development of T H 17 at the expense of T Reg cells.
Stumhofer, J. S. et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunol. 7, 937–945 (2006).
Batten, M. et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunol. 7, 929–936 (2006). References 58 and 59 describe mechanisms by which IL-27 mediates immune suppression, namely, by blocking T H 17 cells.
Racanelli, V., Behrens, S. E., Aliberti, J. & Rehermann, B. Dendritic cells transfected with cytopathic self-replicating RNA induce crosspriming of CD8+ T cells and antiviral immunity. Immunity 20, 47–58 (2004).
Murray, P. J., Aldovini, A. & Young, R. A. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette–Guerin strains that secrete cytokines. Proc. Natl Acad. Sci. USA 93, 934–939 (1996).
Shi, Y., Zheng, W. & Rock, K. L. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc. Natl Acad. Sci. USA 97, 14590–14595 (2000).
Bouwer, H. G., Alberti-Segui, C., Montfort, M. J., Berkowitz, N. D. & Higgins, D. E. Directed antigen delivery as a vaccine strategy for an intracellular bacterial pathogen. Proc. Natl Acad. Sci. USA 103, 5102–5107 (2006).
Happel, K. I. et al. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 73, 5782–5788 (2005).
Antoniou, A. N., Blackwood, S. L., Mazzeo, D. & Watts, C. Control of antigen presentation by a single protease cleavage site. Immunity 12, 391–398 (2000).
Delamarre, L., Couture, R., Mellman, I. & Trombetta, E. S. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J. Exp. Med. 203, 2049–2055 (2006). Describes the impact of lysosomal proteolysis on the immunogenicity of antigens, indicating novel strategies for enhancing vaccine efficacy.
Foulds, K. E., Wu, C. Y. & Seder, R. A. TH1 memory: implications for vaccine development. Immunol. Rev. 211, 58–66 (2006).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Lefrancois, L. & Marzo, A. L. The descent of memory T-cell subsets. Nature Rev. Immunol. 6, 618–623 (2006).
Swain, S. L., Hu, H. & Huston,G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Roberts, A. D., Ely, K. H. & Woodland, D. L. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 202, 123–133 (2005). Describes the contribution over time of effector memory and central memory T cells in pulmonary infection with viruses.
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Dietrich, J. et al. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette–Guerin immunity. J. Immunol. 177, 6353–6360 (2006).
Mollenkopf, H. J. et al. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect. Immun. 72, 6471–6479 (2004).
Goonetilleke, N. P. et al. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette–Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171, 1602–1609 (2003).
Kaufmann, S. H. E. & McMichael, A. J. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nature Med. 11, S33–S44 (2005).
Marrack, P. & Kappler, J. Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 (2004).
Tang, Q. et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 171, 3348–3352 (2003).
Chambers, C. A., Sullivan, T. J. & Allison, J. P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7, 885–895 (1997).
Coyle, A. J. et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 13, 95–105 (2000).
Yoshinaga, S. K. et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402, 827–832 (1999).
Mittrucker, H. W. et al. Inducible costimulator protein controls the protective T cell response against Listeria monocytogenes. J. Immunol. 169, 5813–5817 (2002).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 (1992).
Sharpe, A. H., Wherry, E. J., Ahmed, R. & Freeman, G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunol. 8, 239–245 (2007).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature Immunol. 2, 261–268 (2001).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Brown, J. A. et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170, 1257–1266 (2003).
MacLeod, M. et al. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J. Exp. Med. 203, 897–906 (2006).
Cayabyab, M., Phillips, J. H. & Lanier, L. L. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J. Immunol. 152, 1523–1531 (1994).
McHeyzer-Williams, L. J., Malherbe, L. P. & McHeyzer-Williams, M. G. Helper T cell-regulated B cell immunity. Curr. Top. Microbiol. Immunol. 311, 59–83 (2006).
Tarlinton, D. B-cell memory: are subsets necessary? Nature Rev. Immunol. 6, 785–790 (2006).
Zinkernagel, R. M. & Hengartner, H. Protective 'immunity' by pre-existent neutralizing antibody titers and preactivated T cells but not by so-called 'immunological memory'. Immunol. Rev. 211, 310–319 (2006).
Lanzavecchia, A. et al. Understanding and making use of human memory B cells. Immunol. Rev. 211, 303–309 (2006).
Cushley, W. & Borland, G. in Immunology (eds Kaufmann, S. H. E. & Steward, M. W.) 303–338 (Hodder Arnold/ASM Press, London/Washington, D.C., 2005).
Baumgarth, N., Tung, J. W. & Herzenberg, L. A. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26, 347–362 (2005).
McHeyzer-Williams, L.J., Cool, M. & McHeyzer-Williams, M. G. Antigen-specific B cell memory: expression and replenishment of a novel B220− memory B cell compartment. J. Exp. Med. 191, 1149–1166 (2000).
Kawabe, T. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 (1994).
Ferguson, S. E., Han, S., Kelsoe, G. & Thompson, C. B. CD28 is required for germinal center formation. J. Immunol. 156, 4576–4581 (1996).
Maruyama, M., Lam, K. P. & Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407, 636–642 (2000).
Manz, R. A., Thiel, A. & Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 388, 133–134 (1997).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
O'Connor, B. P., Cascalho, M. & Noelle, R. J. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195, 737–745 (2002).
Pasare, C. & Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 438, 364–368 (2005). Characterizes the role of TLRs in B-cell activation in T-cell antigen-specific antibody responses.
Bernasconi, N. L., Traggiai, E. & Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202 (2002). Explains how antibody production is maintained over long periods of time through polyclonal activation.
Pizza, M. et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287, 1816–1820 (2000).
Kyaw, M. H. et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354, 1455–1463 (2006).
Johri, A. K. et al. Group B Streptococcus: global incidence and vaccine development. Nature Rev. Microbiol. 4, 932–942 (2006).
Projan, S. J., Nesin, M. & Dunman, P. M. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr. Opin. Pharmacol. 6, 473–479 (2006).
Gotz, F. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr. Opin. Microbiol. 7, 477–487 (2004).
McMillan, D. J. & Chhatwal, G. S. Prospects for a group A streptococcal vaccine. Curr. Opin. Mol. Ther. 7, 11–16 (2005).
Olive, C. Progress in M-protein-based subunit vaccines to prevent rheumatic fever and rheumatic heart disease. Curr. Opin. Mol. Ther. 9, 25–34 (2007).
Kotloff, K.L. et al. Safety and immunogenicity of a recombinant multivalent group A streptococcal vaccine in healthy adults: phase 1 trial. JAMA 292, 709–715 (2004).
Maione, D. et al. Identification of a universal Group B streptococcus vaccine by multiple genome screen. Science 309, 148–150 (2005).
Mora, M., Donati, C., Medini, D., Covacci, A. & Rappuoli, R. Microbial genomes and vaccine design: refinements to the classical reverse vaccinology approach. Curr. Opin. Microbiol. 9, 532–536 (2006).
Wizemann, T. M. et al. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69, 1593–1598 (2001).
De Groot, A. S. Immunomics: discovering new targets for vaccines and therapeutics. Drug Discov. Today 11, 203–209 (2006).
Giefing, C., Nagy, E. & von Gabain, A. in Pharmaceutical Biotechnology (eds Guzman, C. A. & Feuerstein, G.) in press (Landes Bioscience, Austin, Texas).
Etz, H. et al. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl Acad. Sci. USA 99, 6573–6578 (2002).
Kuklin, N. A. et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74, 2215–2223 (2006).
Kaufmann, S. H. E. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 26, 660–667 (2005).
Kaufmann, S. H. E. Envisioning future strategies for vaccination against tuberculosis. Nature Rev. Immunol. 6, 699–704 (2006).
Young, D. & Dye, C. The development and impact of tuberculosis vaccines. Cell 124, 683–687 (2006).
Andersen, P. & Doherty, T. M. The success and failure of BCG — implications for a novel tuberculosis vaccine. Nature Rev. Microbiol. 3, 656–662 (2005).
Brockstedt, D. G. et al. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nature Med. 11, 853–860 (2005).
Kursar, M. et al. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178, 2661–2665 (2007).
Brunham, R. C. & Rey-Ladino, J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature Rev. Immunol. 5, 149–161 (2005).
Aebischer, T., Schmitt, A., Walduck, A. K. & Meyer, T. F. Helicobacter pylori vaccine development: facing the challenge. Int. J. Med. Microbiol. 295, 343–353 (2005).
Suerbaum, S. & Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186 (2002).
Baker, M. Anti-infective antibodies: finding the path forward. Nature Biotechnol. 24, 1491–1493 (2006).
Vitetta, E. S. & Ghetie, V. F. Considering therapeutic antibodies. Science 313, 308–309 (2006).
Kim, C. & Kaufmann, S. H. Defensin: a multifunctional molecule lives up to its versatile name. Trends Microbiol. 14, 428–431 (2006).
Kim, C. et al. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl Acad. Sci. USA 102, 4830–4835 (2005). Describes a novel function of human α-defensins, namely, their capacity to neutralize anthrax lethal toxin and, therefore, provides evidence for a novel intervention strategy against anthrax.
Killian, M. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 833–881 (Hodder Arnold, London, 2005).
Sinha, A., Levine, O., Knoll, M. D., Muhib, F. & Lieu, T. A. Cost-effectiveness of pneumococcal conjugate vaccination in the prevention of child mortality: an international economic analysis. Lancet 369, 389–396 (2007).
Mietzner, T. A. & Morse, S. A. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 1270–1300 (Hodder Arnold, London, 2005).
Smith, A. et al. Invasive group A streptococcal disease: should close contacts routinely receive antibiotic prophylaxis? Lancet Infect. Dis. 5, 494–500 (2005).
Bisno, A. L., Brito, M. O. & Collins, C. M. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3, 191–200 (2003).
Areschoug, T., Carlsson, F., Stalhammar-Carlemalm, M. & Lindahl, G. Host–pathogen interactions in Streptococcus pyogenes infections, with special reference to puerperal fever and a comment on vaccine development. Vaccine 22 (Suppl 1), S9–S14 (2004).
Pfyffer, G. E. & Vincent, V. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 1181–1235 (Hodder Arnold, London, 2005).
Märdh, P.-A. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 2006–2025 (Hodder Arnold, London, 2005).
Owen, R. J. in Bacteriology Vol. 2. (eds Borriello, S. P., Murray, P. R. & Funke, G.) 1563–1590 (Hodder Arnold, London, 2005).
Acknowledgements
I am grateful to S. Sibaei and M.L. Grossman for excellent support, D. Schad for graphical work and S. Reece for critically reading the manuscript. I also thank A. von Gabain for sharing unpublished work. Studies on vaccine development have been carried out with support from the following: The European Union Framework Program 6 Projects, Design and Testing of Vaccine Candidates Against Tuberculosis (TBVAC) and Mucosal Vaccines for Poverty-related Diseases (MUVAPRED); the Bundesministerium für Bildung und Forschung KompetenzNetzwerk PathoGenoMikPlus, KompetenzNetzwerk-RNA Technologie, and National Genome Research Network 2 (Germany); DFG Priority Programme Novel Vaccination Strategies (Germany); Bill & Melinda Gates Foundation Grand Challenges in Global Health (US). Studies on the history of vaccinology have been generously supported by Fonds der Chemischen Industrie (Germany).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Stefan Kaufmann is a member of the Scientific Advisory Board of Intercell AG, Austria, and VPM (Vakzine Projekt Management) GmbH, Germany.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Passive vaccination
-
The provision of protective immunity by the transfer of immunoglobulins or T cells.
- Plasma cell
-
A non-dividing, terminally differentiated, immunoglobulin-secreting cell of the B-cell lineage.
- Regulatory T cell
-
(TReg cell). A population of CD4+ T cells that naturally express high levels of CD25 (the interleukin-2 receptor α-chain) and the transcription factor forkhead box P3 (Foxp3), and that have suppressive regulatory activity towards effector T cells and other immune cells.
- Pattern-recognition receptor
-
(PRR). A host receptor (such as Toll-like receptors (TLRs) or NOD-like receptors (NLRs)) that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immune response. These can be membrane-bound (such as TLRs) or soluble cytoplasmic receptors (such as NLRs).
- Dendritic cell
-
(DC). 'Professional' antigen-presenting cells that are found in the T-cell areas of lymphoid tissues and as minor cellular components in most tissues. They have a branched or dendritic morphology and are the most potent stimulators of T-cell responses.
- CD4+ T cell
-
A subpopulation of T cells that express the CD4 receptor. These cells aid in immune responses and are therefore referred to as T helper cells.
- CD8+ T cell
-
A subpopulation of T cells that express the CD8 receptor. CD8+ cells recognize antigens that are presented on the surface of host cells by MHC class I molecules, leading to their destruction, and are therefore also known as cytotoxic T lymphocytes (CTLs).
- γδ T cell
-
A minor population of T cells that express the γδ T-cell receptor, and that are more abundant in epithelial-rich tissues such as skin and gut and reproductive tracts. Like NKT cells, γδ T cells can be cytolytic and produce high levels of cytokines and chemokines.
- Perforin
-
A calcium-sensitive membrane-lytic protein that is found in cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells.
- Granzyme
-
A family of serine proteinases that are found primarily in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. These proteinases enter target cells through perforin pores, then cleave and activate intracellular caspases and induce apoptosis of target cells.
- Phagosome
-
The functional definition of the organelle in which bacteria are internalized. Phagosomal and endosomal pathways undergo interconnected maturation and merge before fusion with lysosomes. Some bacterial pathogens inhibit the acidification of the phagosome and its fusion with lysosomes.
- T helper 1 cell
-
(TH1 cell). A type of activated TH cell that promotes responses associated with the production of a particular set of cytokines, including interleukin (IL)-2 and interferon (IFN)-γ, the main function of which is to stimulate phagocytosis-mediated defences against intracellular pathogens.
- T helper 2 cell
-
(TH2 cell). A type of activated TH cell that participates in phagocytosis-independent responses and downregulates pro-inflammatory responses that are induced by TH1 cells. TH2 cells secrete interleukin (IL)-4 and IL-5.
- Central memory T cell
-
A memory T cell that lacks immediate effector function but can mediate rapid recall responses and has the capacity to circulate from the blood to the secondary lymphoid organs. Rapidly develops the phenotype and function of effector cells after restimulation with antigen.
- Effector memory T cell
-
A memory T cell that homes to inflamed tissues. Can exert immediate effector functions without the need for further differentiation.
- High endothelial venule
-
(HEV). A specialized venule with a cuboidal endothelial lining that occurs in peripheral lymph nodes and Peyer's patches. HEVs allow continuous transmigration of lymphocytes as a consequence of the constitutive expression of adhesion molecules and chemokines at their luminal surface.
- Heterologous prime–boost strategies
-
When a single application of a vaccine is insufficient, repeated vaccinations are carried out using different vaccine preparations, allowing the sequential stimulation of a better immune response.
- Germinal centre
-
A highly specialized and dynamic microenvironment in the follicles of secondary lymphoid tissues (spleen, Peyer's patches and lymph nodes) that gives rise to secondary B-cell follicles during an immune response. The main site of B-cell maturation, leading to the generation of memory B cells and plasma cells that produce high-affinity antibody.
Rights and permissions
About this article
Cite this article
Kaufmann, S. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat Rev Microbiol 5, 491–504 (2007). https://doi.org/10.1038/nrmicro1688
Issue date:
DOI: https://doi.org/10.1038/nrmicro1688
This article is cited by
-
Synthesis, characterization and applications of poly-aliphatic amine dendrimers and dendrons
Journal of the Iranian Chemical Society (2020)
-
A lipidated bi-epitope vaccine comprising of MHC-I and MHC-II binder peptides elicits protective CD4 T cell and CD8 T cell immunity against Mycobacterium tuberculosis
Journal of Translational Medicine (2018)
-
Novel vaccine potential of Rv3131, a DosR regulon-encoded putative nitroreductase, against hyper-virulent Mycobacterium tuberculosis strain K
Scientific Reports (2017)
-
Korrelate für Infektionsschutz nach Impfung
Monatsschrift Kinderheilkunde (2017)
-
A putative nitroreductase from the DosR regulon of Mycobacterium tuberculosis induces pro-inflammatory cytokine expression via TLR2 signaling pathway
Scientific Reports (2016)