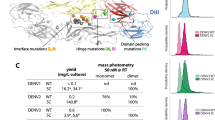
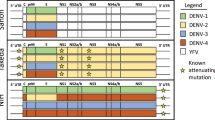
Key Points
-
There are four serotypes of dengue virus (DENV) that are maintained in a human–mosquito–human transmission cycle in most of the tropical and subtropical regions of the world. DENV causes more human disease than any other arbovirus.
-
DENV infection can result in a wide range of outcomes from inapparent infection to dengue fever to potentially fatal dengue haemorrhagic fever (DHF) with shock. The prevalence of severe DENV disease is increasing throughout the world.
-
Infection with DENV elicits a strong neutralizing antibody response against the infecting serotype that is believed to induce lifelong protection against only that particular serotype. DENV-specific T cells are also induced by infection and are believed to aid in the resolution of infection.
-
Conversely, the immune response also appears to be a major factor in the pathogenesis of severe dengue disease. The enhanced disease severity observed in patients undergoing a secondary infection with a virus belonging to a different serotype has been associated with the phenomenon of antibody-dependent enhancement (ADE).
-
An ideal vaccine for DENV must be tetravalent because each of the four serotypes are present throughout the world and each can cause disease. The vaccine should induce neutralizing antibody levels comparable with those observed in wild-type virus infection to limit the risk of ADE. Finally, the vaccine should be available at a low cost for the developing world
-
Multiple live attenuated vaccine candidates are presently being evaluated in clinical trials. Clinical safety and strong immunogenicity have been observed for empirically derived vaccine strains and for recombinant viruses using either genetically modified full-length vaccine strains or antigenic chimeric viruses.
-
Inactivated monovalent vaccines and a recombinant subunit vaccine consisting of purified envelope protein are scheduled to be tested in clinical trials soon. Other approaches are being evaluated preclinically.
-
Considerable progress has been made in the development of DENV vaccine candidates in the past few years. One or more of the strategies being currently pursued should be successful in reducing the disease burden caused by this emerging pathogen.
Abstract
The number of cases of severe dengue disease continues to grow in endemic areas of southeast Asia, Central and South America, and other subtropical regions. Children bear the greatest burden of disease, and the development of an effective vaccine remains a global public health priority. A tetravalent vaccine is urgently needed and must be effective against all four dengue virus serotypes, be cost-effective and provide long-term protection. In this Review we discuss the unique immunological concerns in dengue virus vaccine development and the current prospects for the development of an acceptable vaccine, a goal that is likely to be reached in the near future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burke, D. S. & Monath, T. P. in Fields Virology (eds Knipe, D. M. & Mowley, P. M.) 1043–1125 (Lippincott, Williams & Wilkins, Philadelphia, 2001).
WHO. Dengue haemorrhagic fever: diagnosis, treatment prevention and control (WHO, Geneva, 1997).
Gubler, D. J. & Meltzer, M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 53, 35–70 (1999).
Gubler, D. J. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 1–22 (CAB International, New York, 1997).
Thomas, S. J., Strickman, D. & Vaughn, D. W. Dengue epidemiology: virus epidemiology, ecology, and emergence. Adv. Virus Res. 61, 235–289 (2003).
Mackenzie, J. S., Gubler, D. J. & Petersen, L. R. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Med. 10, S98–S109 (2004). A review that highlights the increasing burden of disease associated with DENV and other flaviviruses.
Rodhain, F. & Rosen, L. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 45–60 (CAB International, New York, 1997).
Effler, P. V. et al. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 11, 742–749 (2005).
Gubler, D. J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11, 480–496 (1998).
Endy, T. P. et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 156, 40–51 (2002). A prospective study describing the range of disease caused by DENV infection.
Guzman, M. G. et al. Epidemiologic studies on dengue in Santiago de Cuba, 1997. Am. J. Epidemiol. 152, 793–799 (2000).
Rush, B. in Medical Inquiries and Observations 89–100 (Prichard and Hall, Philadelphia, 1789).
Halstead, S. B., Yamarat, C. & Scanlon, J. E. The Thai hemorrhagic fever epidemic of 1962: a preliminary report. J. Med. Assoc. Thailand 46, 449–462 (1963).
Burke, D. S., Nisalak, A., Johnson, D. E. & Scott, R. M. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38, 172–180 (1988).
Moreau, J. P., Rosen, L., Saugrain, J. & Lagraulet, J. An epidemic of dengue on Tahiti associated with hemorrhagic manifestations. Am. J. Trop. Med. Hyg. 22, 237–241 (1973).
Sabin, A. B. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1, 30–50 (1952). Review of studies including virus isolation, experimental infection of humans and analysis of homotypic and heterotypic immunity.
Siler, J. F., Hall, M. W. & Hitchens, A. P. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, immunity, and prevention. Philippine J. Sci. 29, 1–304 (1926).
Simmons, J. S., St John, J. H. & Reynolds, F. H. K. Experimental studies of dengue. Philippine J. Sci. 44, 1–252 (1931).
Eram, S. et al. Epidemic dengue hemorrhagic fever in rural Indonesia: II. clinical studies. Am. J. Trop. Med. Hyg. 28, 711–716 (1979).
Halstead, S. B., Nimmannitya, S. & Margiotta, M. R. Dengue and chikungunya virus infection in man in Thailand, 1962–1964: II. Observations on disease in outpatients. Am. J. Trop. Med. Hyg. 18, 972–983 (1969).
Jessie, K., Fong, M. Y., Devi, S., Lam, S. K. & Wong, K. T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189, 1411–1418 (2004).
Lai, P. C. et al. Characteristics of a dengue hemorrhagic fever outbreak in 2001 in Kaohsiung. J. Microbiol. Immunol. Infect. 37, 266–270 (2004).
Halstead, S. B. et al. Observations related to pathogenesis of dengue hemorrhagic fever. I. Experience with classification of dengue viruses. Yale J. Biol. Med. 42, 261–275 (1970).
Halstead, S. B., Scanlon, J., Umpaivit, P. & Udomsakdi, S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964: IV. Epidemiologic studies in the Bangkok metropolitan area. Am. J. Trop. Med. Hyg. 18, 997–1021 (1969).
Innis, B. L. in Kass Handbook of Infectious Diseases: Exotic Virus Infections (ed. Porterfield, J. S.) 103–146 (Chapman & Hall Medical, London, 1995).
Souza, L. J. et al. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz. J. Infect. Dis. 8, 156–163 (2004).
Kalayanarooj, S. et al. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 176, 313–321 (1997).
Kuo, C. H. et al. Liver biochemical tests and dengue fever. Am. J. Trop. Med. Hyg. 47, 265–270 (1992).
Carlos, C. C. et al. Comparison of clinical features and hematologic abnormalities between dengue fever and dengue hemorrhagic fever among children in the Philippines. Am. J. Trop. Med. Hyg. 73, 435–440 (2005).
Nimmannitya, S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 18, 392–397 (1987).
Vaughn, D. W. et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181, 2–9 (2000). This study indicates that increased disease severity was associated with higher viraemia, secondary infection and DENV-2 infection.
Murgue, B., Roche, C., Chungue, E. & Deparis, X. Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996–1997 dengue-2 outbreak in French Polynesia. J. Med. Virol. 60, 432–438 (2000).
Pramulijo, H. S. & Harun, S. R. Ultrasound findings in dengue haemorrhagic fever. Pediatr. Radiol. 21, 100–102 (1991).
Venkata Sai, P. M., Dev, B. & Krishnan, R. Role of ultrasound in dengue fever. Br. J. Radiol. 78, 416–418 (2005).
Subramanian, V., Shenoy, S. & Joseph, A. J. Dengue hemorrhagic fever and fulminant hepatic failure. Dig. Dis. Sci. 50, 1146–1147 (2005).
Patey, O., Ollivaud, L., Breuil, J. & Lafaix, C. Unusual neurologic manifestations occurring during dengue fever infection. Am. J. Trop. Med. Hyg. 48, 793–802 (1993).
Nimmannitya, S., Thisyakorn, U. & Hemsrichart, V. Dengue haemorrhagic fever with unusual manifestations. Southeast Asian J. Trop. Med. Public Health 18, 398–406 (1987).
Kho, L. K., Sumarmo, Wulur, H., Jahja, E. & Gubler, D. J. Dengue hemorrhagic fever accompanied by encephalopathy in Jakarta. Southeast Asian J. Trop. Med. Public Health 12, 83–86 (1981).
Wichmann, O. et al. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop. Med. Int. Health 9, 1022–1029 (2004).
Guzman, M. G. et al. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42, 179–184 (1990).
Halstead, S. B. Etiologies of the experimental dengues of Siler and Simmons. Am. J. Trop. Med. Hyg. 23, 974–982 (1974).
Innis, B. L. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 221–243 (CAB International, Cambridge, 1997).
Calvert, A. E., Huang, C. Y., Kinney, R. M. & Roehrig, J. T. Non-structural proteins of dengue 2 virus offer limited protection to interferon-deficient mice after dengue 2 virus challenge. J. Gen. Virol. 87, 339–346 (2006).
Samuel, M. A. & Diamond, M. S. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80, 9349–9360 (2006).
Bukowski, J. F. et al. Dengue virus-specific cross-reactive CD8+ human cytotoxic T lymphocytes. J. Virol. 63, 5086–5091 (1989).
Kurane, I., Meager, A. & Ennis, F. A. Dengue virus-specific human T cell clones. Serotype crossreactive proliferation, interferon γ production, and cytotoxic activity. J. Exp. Med. 170, 763–775 (1989).
Halstead, S. B. et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg. Infect. Dis. 8, 1474–1479 (2002).
Kliks, S. C., Nimmannitya, S., Nisalak, A. & Burke, D. S. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38, 411–419 (1988).
Pengsaa, K. et al. Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in thai children. J. Infect. Dis. 194, 1570–1576 (2006). References 48 and 49 demonstrate the presence of DENV-specific maternal antibodies in infants and suggest that they are protective until their levels decrease below a protective threshold.
Kaufman, B. M. et al. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41, 576–580 (1989).
Kaufman, B. M., Summers, P. L., Dubois, D. R. & Eckels, K. H. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36, 427–434 (1987).
Crill, W. D. & Roehrig, J. T. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75, 7769–7773 (2001).
Roehrig, J. T., Bolin, R. A. & Kelly, R. G. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246, 317–328 (1998).
Roehrig, J. T. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 199–220 (CAB International, New York, 1997).
Blaney, J. E., Jr, Matro, J. M., Murphy, B. R. & Whitehead, S. S. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J. Virol. 79, 5516–5528 (2005).
Sun, W. et al. Vaccination of human volunteers with monovalent and tetravalent live-attenuated dengue vaccine candidates. Am. J. Trop. Med. Hyg. 69, 24–31 (2003).
Edelman, R. et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am. J. Trop. Med. Hyg. 69, 48–60 (2003). References 56 and 57 describe clinical studies of the empirically derived live attenuated DENV vaccines characterized by the United States Army.
Stephens, H. A. et al. HLA-A and-B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60, 309–318 (2002).
Sakuntabhai, A. et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nature Genet. 37, 507–513 (2005).
Fernandez-Mestre, M. T., Gendzekhadze, K., Rivas-Vetencourt, P. & Layrisse, Z. TNF-α-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64, 469–472 (2004).
Gubler, D. J., Suharyono, W., Lubis, I., Eram, S. & Gunarso, S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am. J. Trop. Med. Hyg. 30, 1094–1099 (1981).
Gubler, D. J., Reed, D., Rosen, L. & Hitchcock, J. C. Jr. Epidemiological, clinical, and virologic observations on dengue in The Kingdom of Tonga. Am. J. Trop. Med. Hyg. 27, 581–589 (1978).
Guzman, M. G. et al. Clinical and serologic study of Cuban children with dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). Bull. Pan Am. Health Organ. 21, 270–279 (1987).
Halstead, S. B., Nimmannitya, S. & Cohen, S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 42, 311–328 (1970).
Halstead, S. B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60, 421–467 (2003). A review that discusses the evidence supporting the role of antibody-dependent enhancement in the enhanced severity of DENV infection associated with secondary infection.
Halstead, S. B. Pathogenesis of dengue: challenges to molecular biology. Science 239, 476–481 (1988).
Kurane, I. & Ennis, F. A. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 273–290 (CAB International, New York, 1997).
Nguyen, T. H. et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J. Infect. Dis. 189, 221–232 (2004).
Green, S. & Rothman, A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19, 429–436 (2006). A broad review of the immunopathological mechanisms that are associated with DENV infection and how they can result in the symptoms of severe disease.
Kliks, S. C., Nisalak, A., Brandt, W. E., Wahl, L. & Burke, D. S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40, 444–451 (1989).
Stephenson, J. R. Understanding dengue pathogenesis: implications for vaccine design. Bull. World Health Organ. 83, 308–314 (2005).
Avirutnan, P. et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 193, 1078–1088 (2006).
Blaney, J. E. Jr, Durbin, A. P., Murphy, B. R. & Whitehead, S. S. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 19, 10–32 (2006). A summary of the preclinical and clinical progress towards developing a recombinant, tetravalent DENV vaccine at NIAID.
Guirakhoo, F. et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum. Vaccin. 2, 60–67 (2006). The first reported clinical trial of a monovalent ChimeriVax DENV vaccine candidate.
Kanesa-thasan, N. et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 19, 3179–3188 (2001).
Sabchareon, A. et al. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr. Infect. Dis. J. 23, 99–109 (2004).
Kitchener, S. et al. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine 24, 1238–1241 (2006).
Butrapet, S. et al. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J. Virol. 74, 3011–3019 (2000).
Sanchez, V. et al. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3). Vaccine 24, 4914–4926 (2006).
Men, R., Bray, M., Clark, D., Chanock, R. M. & Lai, C. J. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J. Virol. 70, 3930–3937 (1996).
Whitehead, S. S. et al. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 77, 1653–1657 (2003).
Blaney, J. E. Jr, Hanson, C. T., Hanley, K. A., Murphy, B. R. & Whitehead, S. S. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 4, 39 (2004).
Blaney, J. E. Jr et al. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am. J. Trop. Med. Hyg. 71, 811–821 (2004).
Durbin, A. P. et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum. Vaccin. 2, 167–173 (2006).
Durbin, A. P. et al. rDEN4 Δ30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J. Infect. Dis. 191, 710–718 (2005).
Durbin, A. P. et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 65, 405–413 (2001).
Troyer, J. M. et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am. J. Trop. Med. Hyg. 65, 414–419 (2001).
Whitehead, S. S. et al. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine 21, 4307–4316 (2003).
Durbin, A. P. et al. rDEN2/4D30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum. Vaccin. 2, 255–260 (2006).
Guirakhoo, F. et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 75, 7290–7304. (2001).
Higgs, S. et al. Growth characteristics of ChimeriVax-Den vaccine viruses in Aedes aegypti and Aedes albopictus from Thailand. Am. J. Trop. Med. Hyg. 75, 986–993 (2006).
Guirakhoo, F. et al. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298, 146–159 (2002).
Huang, C. Y. et al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J. Virol. 77, 11436–11447 (2003). This study describes using recombinant technology to produce antigenic chimeric vaccine candidates in the DENV-2 PDK-53 vaccine genetic background.
Simmons, M., Porter, K. R., Hayes, C. G., Vaughn, D. W. & Putnak, R. Characterization of antibody responses to combinations of a dengue virus type 2 DNA vaccine and two dengue virus type 2 protein vaccines in rhesus macaques. J. Virol. 80, 9577–9585 (2006).
Putnak, R. et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J. Infect. Dis. 174, 1176–1184 (1996).
Putnak, R. et al. Immunogenic and protective response in mice immunized with a purified, inactivated, Dengue-2 virus vaccine prototype made in fetal rhesus lung cells. Am. J. Trop. Med. Hyg. 55, 504–510 (1996).
Putnak, R. et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine 23, 4442–4452 (2005).
Guzman, M. G. et al. Induction of neutralizing antibodies and partial protection from viral challenge in Macaca fascicularis immunized with recombinant dengue 4 virus envelope glycoprotein expressed in Pichia pastoris. Am. J. Trop. Med. Hyg. 69, 129–134 (2003).
Raviprakash, K. et al. Needle-free Biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology 315, 345–352 (2003).
Deen, J. L. et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet 368, 170–173 (2006). A discussion of the issues surrounding the WHO case definition of DHF and its possible role in under-reporting of severe disease.
Lindenbach, B. D. & Rice, C. M. in Fields Virology (eds Knipe, D. M. & Howley, P. M.) 1043–1125 (Lippincott Williams and Wilkins, Philadelphia, 2001).
Clyde, K. & Harris, E. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J. Virol. 80, 2170–2182 (2006).
Mukhopadhyay, S., Kuhn, R. J. & Rossmann, M. G. A structural perspective of the flavivirus life cycle. Nature Rev. Microbiol. 3, 13–22 (2005). A clear review of the flavivirus virion structure and the dynamics of virion formation.
Kuhn, R. J. et al. Structure of dengue virus. Implications for flavivirus organization, maturation, and fusion. Cell 108, 717–725 (2002).
Bray, M. et al. Mice immunized with recombinant vaccinia virus expressing dengue 4 virus structural proteins with or without nonstructural protein NS1 are protected against fatal dengue virus encephalitis. J. Virol. 63, 2853–2856 (1989).
Schlesinger, J. J., Brandriss, M. W., Cropp, C. B. & Monath, T. P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 60, 1153–1155 (1986).
Russell, P. K., Nisalak, A., Sukhavachana, P. & Vivona, S. A plaque reduction test for dengue virus neutralization antibodies. J. Immunol. 99, 285–290 (1967).
Laoprasopwattana, K. et al. Dengue virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J. Infect. Dis. 192, 510–519 (2005).
Garcia, G. et al. Antibodies from patients with dengue viral infection mediate cellular cytotoxicity. J. Clin. Virol. 37, 53–57 (2006).
Falgout, B., Bray, M., Schlesinger, J. J. & Lai, C. J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 64, 4356–4363 (1990).
Sabchareon, A. et al. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am. J. Trop. Med. Hyg. 66, 264–272 (2002).
Mongkolsapaya, J. et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nature Med. 9, 921–927 (2003).
Munoz-Jordan, J. L. et al. Inhibition of α/β interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79, 8004–8013 (2005).
Diallo, M. et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999–2000: entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 9, 362–367 (2003).
Wolfe, N. D. et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 64, 310–316 (2001).
Sun, W. et al. Protection of Rhesus monkeys against dengue virus challenge after tetravalent live attenuated dengue virus vaccination. J. Infect. Dis. 193, 1658–1665 (2006).
Acknowledgements
Support for S.S.W., J.E.B., A.P.D. (in part) and B.R.M. is provided by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The intellectual property for which the authors are listed as inventors has been non-exclusively licensed to numerous private companies.
Related links
Related links
DATABASES
Entrez Genome
Entrez Genome Project
FURTHER INFORMATION
Laboratory of Infectious Diseases
Glossary
- Sylvatic
-
A form of disease that occurs in wild animals.
- Dendritic cells
-
A 'professional' antigen-presenting cell that is found in the T-cell areas of lymphoid tissues and as a minor cellular component in most tissues, including skin. They have a branched or dendritic morphology and are the most potent stimulators of T-cell responses.
- Hypovolemia
-
A decrease in intravascular number.
- Ascites
-
The accumulation of fluid in the peritoneal cavity.
- Councilman bodies
-
An eosinophilic globule seen in the liver of individuals with viral haemorrhagic fevers, especially yellow fever. Councilman bodies result from necrosis of a single liver cell.
- Adaptive immune response
-
Represented by B and T lymphocytes that express antigen-specific receptors. Memory lymphocytes persist, providing immunity against re-infection.
- Parenteral route
-
A route other than the gastrointestinal route, such as intravenous, subcutaneous or intradermal.
- Reverse genetic technique
-
A method that allows the production of viruses from cloned cDNA.
- Prime–boost strategy
-
When a single application of a vaccine is insufficient, repeated immunizations are carried out with either the same vaccine preparation (homologous prime–boost) or different vaccine preparations (heterologous prime–boost) to sequentially stimulate a better immune response.
Rights and permissions
About this article
Cite this article
Whitehead, S., Blaney, J., Durbin, A. et al. Prospects for a dengue virus vaccine. Nat Rev Microbiol 5, 518–528 (2007). https://doi.org/10.1038/nrmicro1690
Issue date:
DOI: https://doi.org/10.1038/nrmicro1690
This article is cited by
-
Dengue in the urban slums of Pakistan: health costs, adaptation practices, and the role of dengue-diagnosis and surveillance in controlling the epidemic
GeoJournal (2024)
-
Neutralizing antibodies targeting a novel epitope on envelope protein exhibited broad protection against flavivirus without risk of disease enhancement
Journal of Biomedical Science (2023)
-
Development of flavivirus subviral particles with low cross-reactivity by mutations of a distinct antigenic domain
Applied Microbiology and Biotechnology (2023)
-
Stem cell therapy: a novel approach against emerging and re-emerging viral infections with special reference to SARS-CoV-2
Molecular Biology Reports (2023)
-
Human seminal virome: a panel based on recent literature
Basic and Clinical Andrology (2022)