Abstract
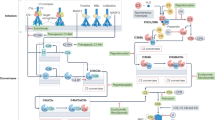
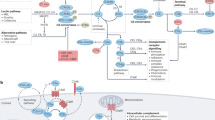
The complement system has vital protective functions as a humoral component of the innate immune system and also through interactions with the adaptive immune system; however, when inappropriately activated or regulated, complement can cause inflammation and organ damage, and such processes are involved in the pathogenesis of many inflammatory conditions, not least rheumatic diseases. Furthermore, states of complement deficiency can predispose not only to infections, but also to autoimmune disorders, including rheumatic diseases such as systemic lupus erythematosus. In this Review, the mechanisms behind the pathogenic activities of complement in rheumatic diseases are discussed. Potential approaches to therapeutic intervention that focus on regulating complement activities in these disorders are also considered.
Key Points
-
Complement activation is involved in the pathogenesis of many rheumatic diseases
-
Deficiencies in components of the classical pathway of complement activation, but not other complement deficiencies, are associated with development of systemic lupus erythematosus (SLE) and SLE-like disorders
-
Complement activation via the alternative pathway in tissues can cause damage associated with autoimmune disease pathology
-
Increased knowledge of the functions of complement will hopefully lead to identification of new therapeutic targets in rheumatic diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
29 June 2012
In the version of this article initially published online, Figure 1 incorrectly showed an arrow from C7 to C5b. This should have been a dashed line. The error has been corrected for the print, HTML and PDF versions of the article.
References
Medof, M. E., Iida, K., Mold, C. & Nussenzweig, V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J. Exp. Med. 156, 1739–1754 (1982).
Fearon, D. T. & Carter, R. H. The CD19/CR2/TAPA-1 complex of B-lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13, 127–149 (1995).
Carroll, M. C. & Prodeus, A. P. Linkages of innate and adaptive immunity. Curr. Opin. Immunol. 10, 36–40 (1998).
Heeger, P. S. & Kemper, C. Novel roles of complement in T effector cell regulation. Immunobiology 217, 216–224 (2011).
Müller-Eberhard, H. J. Molecular organization and function of the complement system. Annu. Rev. Biochem. 57, 321–347 (1988).
Carroll, M. C. The lupus paradox. Nat. Genet. 19, 3–4 (1998).
Skattum, L., van Deuren, M., van der Poll, T. & Truedsson, L. Complement deficiency states and associated infections. Mol. Immunol. 48, 1643–1655 (2011).
Truedsson, L., Bengtsson, A. A. & Sturfelt, G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40, 560–566 (2007).
Garred, P., Voss, A., Madsen, H. O. & Junker, P. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes Immun. 2, 442–450 (2001).
Jönsen, A. et al. Genetically determined mannan-binding lectin deficiency is of minor importance in determining susceptibility to severe infections and vascular organ damage in systemic lupus erythematosus. Lupus 16, 245–253 (2007).
Lipsker, D. M. et al. Lupus erythematosus associated with genetically determined deficiency of the second component of the complement. Arch. Dermatol. 136, 1508–1514 (2000).
Racila, D. M. et al. Homozygous single nucleotide polymorphism of the complement C1QA gene is associated with decreased levels of C1q in patients with subacute cutaneous lupus erythematosus. Lupus 12, 124–132 (2003).
Yu, C. Y. & Whitacre, C. C. Sex, MHC and complement C4 in autoimmune diseases. Trends Immunol. 25, 694–699 (2004).
Law, S. K. & Dodds, A. W. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 6, 263–274 (1997).
Sturfelt, G. et al. Homozygous C4A deficiency in systemic lupus erythematosus—analysis of patients from a defined population. Clin. Genet. 38, 427–433 (1990).
Davies, K. E., Peters, A. M., Beynon, H. L. & Walport, M. J. Immune complex processing in patients with systemic lupus erythematosus. In vivo imaging and clearance studies. J. Clin. Invest. 90, 2075–2083 (1992).
Sturfelt, G., Nived, O. & Sjöholm, A. G. Kinetic analysis of immune complex solubilization: complement function in relation to disease activity in SLE. Clin. Exp. Rheumatol. 10, 241–247 (1992).
Carroll, M. C. The role of complement in B cell activation and tolerance. Adv. Immunol. 74, 61–88 (2000).
Korb, L. C. & Ahearn, J. M. C1q binds directly and specifically to surface blebs of human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 158, 4525–4528 (1997).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19, 56–59 (1998).
Gullstrand, B., Mårtensson, U., Sturfelt, G., Bengtsson, A. A. & Truedsson, L. Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells. Clin. Exp. Immunol. 156, 303–311 (2009).
Yamada, M. et al. Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur. J. Immunol. 34, 221–230 (2004).
Lood, C. et al. C1q inhibits immune-complex induced interferon-α production in plasmacytoid dendritic cells. Arthritis Rheum. 60, 3081–3090 (2009).
Lövgren, T., Eloranta, M. L., Båve, G., Alm, G. V. & Rönnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Molina, H. Update on complement in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Rheumatol. 14, 492–497 (2002).
Shlomchik, M. J. & Madaio, M. P. The role of antibodies and B cells in the pathogenesis of lupus nephritis. Springer Semin. Immunopathol. 24, 363–375 (2003).
Mostoslavsky, G. et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur. J. Immunol. 31, 1221–1227 (2001).
Kalaaji, M., Sturfelt, G., Mjelle, J. E., Nossent, H. & Rekvig, O. P. Critical comparative analyses of anti-α-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 54, 914–926 (2006).
Kalaaji, M. et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 71, 664–672 (2007).
Schwartz, M. M., Korbet, S. M. & Lewis, E. J. The prognosis and pathogenesis of severe lupus glomerulonephritis. Nephrol. Dial. Transplant. 23, 1298–1306 (2008).
Sturfelt, G. & Sjöholm, A. G. Complement components, complement activation and acute phase response in systemic lupus erythematosus. Int. Arch. Allergy Appl. Immunol. 75, 75–83 (1984).
Najafi, C. C., Korbet, S. M., Schwartz, M. M., Reichlin, M. & Evans, J. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int. 59, 2156–5163 (2001).
Sjöholm, A. G., Mårtensson, U. & Sturfelt, G. Serial analysis of autoantibody responses to the collagen-like region of Clq, collagen type II, and double stranded DNA in patients with systemic lupus erythematosus. J. Rheumatol. 24, 871–878 (1997).
Antes, U., Heinz, H. P. & Loos, M. Evidence for the presence of autoantibodies to the collagen-like portion of C1q in systemic lupus erythematosus. Arthritis Rheum. 31, 457–464 (1988).
Trouw, L. A. & Daha, M. R. Role of anti-C1q antibodies in the pathogenesis of lupus nephritis. Expert Opin. Biol. Ther. 5, 243–251 (2005).
Bigler, C., Schaller, M., Perahud, I., Osthoff, M. & Trendelenburg, M. Autoantibodies against complement C1q specifically target C1q bound on early apoptotic cells. J. Immunol. 183, 3512–3521 (2009).
Trouw, L. A., Seelen, M. A. & Daha, M. R. Complement and renal disease. Mol. Immunol. 40, 125–134 (2003).
Pickering, M. C. et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31, 424–428 (2002).
Nielsen, C. T. et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 64, 1227–1236 (2012).
Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I. & Simon, H. U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444 (2009).
Hakkim, A. et al. Impairment of neutrophil trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13–BLK and ITGAM–ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Jacob, H. S., Craddock, P. R., Hammerschmidt, D. E. & Moldow, C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N. Engl. J. Med. 302, 789–794 (1980).
Abrahamson, S. B., Given, W. P., Edelson, H. S. & Weissmann, G. Neutrophil aggregation induced by sera from patients with active systemic lupus erythematosus. Arthritis Rheum. 26, 630–636 (1983).
Karpman, D. & Kahn, R. The contact/kinin and complement systems in vasculitis. APMIS Suppl. 127, 48–54 (2009).
Ghiran, I. C. et al. Systemic lupus erythematosus serum deposits C4d on red blood cells, decreases red blood cell membrane deformability and promotes nitric oxide production. Arthritis Rheum. 63, 503–512 (2011).
Gewurz, H., Ying, S. C., Jiang, H. & Lint, T. F. Nonimmune activation of the classical complement pathway. Behring Inst. Mitt. 93, 138–147 (1993).
Sjöwall, C. et al. Solid-phase classical complement activation by C-reactive protein (CRP) is inhibited by fluid-phase CRP-C1q interaction. Biochem. Biophys. Res. Commun. 352, 251–258 (2007).
Rodriguez, W. et al. Prevention and reversal of nephritis in MRL/lpr mice with a single injection of C-reactive protein. Arthritis Rheum. 54, 325–335 (2006).
Carlucci, F., Cool, H. T., Garg, A., Pepys, M. B. & Botto, M. Lack of effect of a single injection of human C-reactive protein on murine lupus or nephrotoxic nephritis. Arthritis Rheum. 62, 245–249 (2010).
Lim, W. Complement and the antiphospholipid syndrome. Curr. Opin. Hematol. 18, 361–365 (2011).
Holers, V. M. et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 21, 211–220 (2002).
Jönsson, G. et al. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology (Oxford) 46, 1133–1139 (2007).
Welch, T. R., Frenzke, M., Witte, D. & Davis, A. E. 3rd. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin. Exp. Immunol. 130, 43–48 (2002).
Bao, L., Haas, M. & Quigg, R. J. Complement factor H deficiency accelerates development of lupus nephritis. J. Am. Soc. Nephrol. 22, 285–295 (2011).
Jönsen, A. et al. Mutations in genes encoding complement inhibitors CD46 and CFH affect the age at nephritis onset in patients with systemic lupus erythematosus. Arthritis Res. Ther. 13, R206 (2011).
Burnham, T. K., Neblett, T. R. & Fine, G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J. Invest. Dermatol. 41, 451–456 (1963).
Casciola-Rosen, L. & Rosen, A. Ultraviolet light-induced keratinocyte apoptosis: a potential mechanism for the induction of skin lesions and autoantibody production in LE. Lupus 6, 175–180 (1997).
Tveita, A. A., Ninomiya, Y., Sado, Y., Rekvig, O. P. & Zykova, S. N. Development of lupus nephritis is associated with qualitative changes in the glomerular collagen IV matrix composition. Lupus 18, 355–360 (2009).
Petri, M., Watson, R., Winkelstein, J. A. & McLean, R. H. Clinical expression of systemic lupus erythematosus in patients with C4A deficiency. Medicine (Baltimore) 72, 236–244 (1993).
Sturfelt, G., Sjöholm, A. G. & Svensson, B. Complement components, C1 activation and disease activity in SLE. Int. Arch. Allergy Appl. Immunol. 70, 12–18 (1983).
Zadura, F. A., Theander, E., Blom, A. M. & Trouw, L. A. Complement inhibitor C4b-binding protein in primary Sjögren's syndrome and its association with other disease markers. Scand. J. Immunol. 69, 374–380 (2009).
Ghererdi, R. K. Pathogenic aspects of dermatomyositis, polymyositis and overlap myositis. Presse Med. 40, 209–218 (2011).
Scambi, C. et al. Comparative proteomic analysis of serum from patients with systemic sclerosis and sclerodermatous GVHD. Evidence of defective function of factor H. PLoS One 5, e12162 (2010).
Trouw, L. A. et al. Genetic variants of C1q are a risk for rheumatoid arthritis. Ann. Rheum. Dis. 70 (Suppl. 2), A17 (2011).
Hedberg, H. The depressed synovial complement activity in adult and juvenile rheumatoid arthritis. Acta Rheum. Scand. 10, 109–127 (1964).
Pekin, T. J. Jr & Zvaifler, N. J. Hemolytic complement in synovial fluid Clin. Invest. 43, 1372–1382 (1964).
Hedberg, H., Lundh, B. & Laurell, A. B. Studies on the third component of complement in synovial fluid from arthritic patients. II. Conversion and its relation to total complement. Clin. Exp. Immunol. 6, 707–712 (1970).
Sjöholm, A. G., Berglund, K., Johnson, U., Laurell, A. B. & Sturfelt, G. C1 activation, with C1q in excess of functional C1 in synovial fluid from patients with rheumatoid arthritis. Int. Arch. Allergy Appl. Immunol. 79, 113–119 (1986).
Okroj, M., Heinegård, D., Holmdahl, R. & Blom, A. M. Rheumatoid arthritis and the complement system. Ann. Med. 39, 517–530 (2007).
Nandakumar, K. S. et al. A recombinant vaccine effectively induces C5a-specific neutralizing antibodies and prevents arthritis. PLoS One 5, e13511 (2010).
Konttinen, Y. T. et al. Complement in acute and chronic arthritides: assessment of C3c, C9 and protectin (CD59) in synovial membrane. Ann. Rheum. Dis. 55, 888–894 (1996).
Jahn, B., von Kempis, J., Krämer, K. L., Filsinger, S. & Hänsch, G. M. Interaction of the terminal complement components C5b–9 with synovial fibroblasts: binding to the membrane surface leads to increased levels in collagenase-specific mRNA. Immunology 78, 329–334 (1993).
Banda, N. K. et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induce arthrits in mice. J. Immunol. 188, 1469–1478 (2012).
Berckmans, R. J. et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor XII-dependent mechanism. Arthritis Rheum. 46, 2857–2866 (2002).
Berglund, K., Laurell, A. B., Nived, O., Sjöholm, A. G. & Sturfelt, G. Complement activation, circulating C1q binding substances and inflammatory activity in rheumatoid arthritis: relations and changes on suppression of inflammation. J. Clin. Lab. Immunol. 4, 7–14 (1980).
Brodeur, J. P., Ruddy, S., Schwartz, L. B. & Moxley, G. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 34, 1531–1537 (1991).
Hermansson, M. et al. MS analysis of rheumatoid arthritic synovial tissue identifies specific citrullination sites on fibrinogen. Proteomics Clin. Appl. 4, 511–518 (2010).
Trouw, L. A. et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patient activate complement via both the classical and the alternative pathways. Arthritis Rheum. 60, 1923–1931 (2009).
Hanauske-Abel, H. M., Pontz, B. F. & Schorlemmer, H. U. Cartilage specific collagen activates macrophages and the alternative pathway of complement: evidence of an immunopathogenetic concept of rheumatoid arthritis. Ann. Rheum. Dis. 41, 168–176 (1982).
Schaapherder, A. F., Gooszen, H. G., te Bulte, M. T. & Daha, M. R. Human complement activation via the alternative pathway on porcine endothelium initiated by IgA antibodies. Transplantation 60, 287–291 (1995).
Kilpatrick, D. C. Mannan-binding lectin and its role in innate immunity. Transfus. Med. 12, 335–352 (2002).
Sjöberg, A., Önnerfjord, P., Mörgelin, M., Heinegård, D. & Blom, A. M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308 (2005).
Happonen, K. E., Sjöberg, A. P., Mörgelin, M., Heinegård, D. & Blom, A. M. Complement inhibitor C4b-binding protein interacts directly with small glycoproteins of the extracellular matrix. J. Immunol. 182, 1518–1525 (2009).
Groeneveld, T. W. et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J. Immunol. 175, 4715–4723 (2005).
Happonen, K. E. et al. Regulation of complement by cartilage oligomeric matrix protein (COMP) allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 62, 3574–3583 (2010).
Turesson, C. & Matteson, E. L. Vasculitis in rheumatoid arthritis. Curr. Opin. Rheumatol. 21, 35–40 (2009).
Geirsson, A. J., Sturfelt, G. & Truedsson, L. Clinical and serological features of severe vasculitis in rheumatoid arthritis: prognostic implications. Ann. Rheum. Dis. 46, 727–733 (1987).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Xiao, H. et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
Huugen, D. et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 71, 646–654 (2007).
Kose, A. A. Direct immunofluorescence in Behçet's disease: a controlled study with 108 cases. Yonsei Med. J. 50, 505–511 (2009).
Mårtensson, U., Sjöholm, A. G., Sturfelt, G., Truedsson, L. & Laurell, A. B. Western blot analysis of human IgG reactive with the collagenous portion of C1q: evidence of distinct binding specificities. Scand. J. Immunol. 35, 735–744 (1992).
Mehregan, D. R. & Gibson, L. E. Pathophysiology of urticarial vasculitis. Arch. Dermatol. 134, 88–89 (1998).
Emlen, W., Li, W. & Kirschfink, M. Therapeutic complement inhibition: new developments. Semin. Thromb. Hemost. 36, 660–668 (2010).
Longhurst, H. & Cicardi, M. Hereditary angio-oedema. Lancet 379, 474–481 (2012).
Garred, P. et al. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr. Pulmonol. 33, 201–207 (2002).
Mehta, P. et al. SLE with C1q deficiency treated with fresh frozen plasma: a 10-year experience. Rheumatology (Oxford) 49, 823–824 (2010).
Steinsson, K., Erlendsson, K. & Valdimarsson, H. Successful plasma infusion treatment of a patient with C2 deficiency and systemic lupus erythematosus: clinical experience over forty-five months. Arthritis Rheum. 32, 906–913 (1989).
Weisman, H. F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990).
Jennette, J. C., Xiao, H., Falk, R. & Gasim, A. M. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic antibodies. Contrib. Nephrol. 169, 211–220 (2011).
Macor, P. et al. Treatment of arthritis models by targeting synovial endothelium with a neutralizing recombinant antibody to C5. Arthritis Rheum. http://dx.doi.org/10.1002/art.34430.
Kolla, R. V. et al. Complement C3d conjugation to anthrax protective antigen promotes a rapid, sustained, and protective antibody response. PLoS One 2, e1044 (2007).
Wu, Y. L., Brookshire, B. P., Verani, R. R., Arnett, F. C. & Yu, C. Y. Clinical presentations and molecular basis of complement C1r deficiency in a male African-American patient with systemic lupus erythematosus. Lupus 20, 1126–1134 (2011).
Fijen, C. A. et al. Properdin deficiency: molecular basis and disease association. Mol. Immunol. 36, 863–867 (1999).
Witzel-Schlömp, K. et al. Heterogeneity in the genetic basis of human complement C9 deficiency. Immunogenetics 48, 144–147 (1998).
Author information
Authors and Affiliations
Contributions
G. Sturfelt and L. Truedsson contributed equally to researching data for the article, discussions of the content, writing the article and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G. Sturfelt has acted as a consultant for Active Biotech. L. Truedsson declares no competing interests.
Rights and permissions
About this article
Cite this article
Sturfelt, G., Truedsson, L. Complement in the immunopathogenesis of rheumatic disease. Nat Rev Rheumatol 8, 458–468 (2012). https://doi.org/10.1038/nrrheum.2012.75
Published:
Issue date:
DOI: https://doi.org/10.1038/nrrheum.2012.75
This article is cited by
-
Cross-reactivity of IgM anti-modified protein antibodies in rheumatoid arthritis despite limited mutational load
Arthritis Research & Therapy (2021)
-
Production of a bispecific antibody targeting TNF-α and C5a in Pichia pastoris and its therapeutic potential in rheumatoid arthritis
Biotechnology Letters (2020)
-
Two subgroups in systemic lupus erythematosus with features of antiphospholipid or Sjögren’s syndrome differ in molecular signatures and treatment perspectives
Arthritis Research & Therapy (2019)
-
The involvement of C5a in the progression of experimental arthritis with Porphyromonas gingivalis infection in SKG mice
Arthritis Research & Therapy (2018)
-
The complement system as a potential therapeutic target in rheumatic disease
Nature Reviews Rheumatology (2017)