Abstract
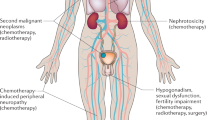
The most common childhood genitourinary cancers are Wilms tumour, rhabdomyosarcoma and germ cell tumour (GCT). Long-term survival rates for patients with these tumours are generally excellent, ranging from 80% to 100%. However, the high cure rates have highlighted the need to minimize the long-term complications of treatments (referred to as 'late effects'), which can be caused by the three treatment modalities used to treat genitourinary tumours: surgery, chemotherapy and radiation therapy. Serious late effects, such as death, second cancers and tumour recurrence, are uncommon but do occur occasionally. Chronic health conditions—such as cardiac, pulmonary and fertility disorders—are more prevalent. Given the high prevalence of late effects, survivors of childhood genitourinary malignancies require regular surveillance and health promotion delivered by health-care providers with specialist knowledge of the long-term complications of treatment.
Key Points
-
Overall survival rates for children with Wilms tumour, genitourinary rhabdomyosarcoma and gonadal germ cell tumours are 80–100%
-
Serious late effects, such as death, second malignancies and recurrence are uncommon in survivors of genitourinary cancers, but chronic health conditions often arise
-
Given the high incidence and prevalence of late effects, survivors of childhood genitourinary malignancies require regular disease surveillance and health promotion delivered by healthcare providers knowledgeable in this area
-
Continued research is needed to understand the mechanisms of late effects and to, therefore, reduce long-term complications from treatments of childhood genitourinary malignancies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Howlader, N. et al. (eds). SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. Surveillance, Epidemiology and End Results [online], (2012).
Wakefield, C. E. et al. The psychosocial impact of completing childhood cancer treatment: a systematic review of the literature. J. Pediatr. Psychol. 35, 262–274 (2010).
Zeltzer, L. K. et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 27, 2396–2404 (2009).
Breslow, N. et al. Age distribution of Wilms' tumor: Report from the National Wilms' Tumor Study. Cancer Res. 48, 1653–1657 (1988).
Metzger, M. L. & Dome, J. S. Current therapy for Wilms' tumor. Oncologist 10, 815–826 (2005).
Murphy, G. P., Williams, P. D. & Klein, R. The growth characteristics of the metastatic Wistar/Furth Wilms' tumor model. Res. Commun. Chem. Pathol. Pharmacol. 12, 397–404 (1975).
Grundy, P. E. et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J. Clin. Oncol. 23, 7312–7321 (2005).
Dome, J. S. et al. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J. Clin. Oncol. 24, 2352–2358 (2006).
Hamilton, T. E. et al. The management of synchronous bilateral Wilms tumor: a report from the National Wilms Tumor Study Group. Ann. Surg. 253, 1004–1010 (2011).
Termuhlen, A. M. et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 57, 1210–1216 (2011).
Cotton, C. A. et al. Early and late mortality after diagnosis of wilms tumor. J. Clin. Oncol. 27, 1304–1309 (2009).
Green, D. M. et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J. Clin. Oncol. 7, 1926–1934 (2001).
Breslow, N. E. et al. Second malignant neoplasms following treatment for Wilm's tumor: a report from the National Wilms' Tumor Study Group. J. Clin. Oncol. 13, 1851–1859 (1995).
Breslow, N. E. et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int. J. Cancer 3, 657–666 (2010).
Green, D. M. et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J. Clin. Oncol. 27, 2374–2381 (2009).
Kalapurakal, J. A. et al. Pregnancy outcomes after abdominal irradiation that included or excluded the pelvis in childhood Wilms tumor survivors: a report from the National Wilms Tumor Study. Int. J. Radiat. Oncol. Biol. Phys. 58, 1364–1368 (2004).
Green, D. M. et al. Pregnancy outcome after treatment for Wilms tumor: a report from the national Wilms tumor long-term follow-up study. J. Clin. Oncol. 28, 2824–2830 (2010).
Green, D. M. et al. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J. Clin. Oncol. 20, 2506–2513 (2002).
Lange, J. et al. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J. Urol. 86, 378–386 (2011).
Gurney, J. G., Young, Jr, J. L., Roffers, S. D., Smith, M. A. & Buni, G. R. Soft Tissue Sarcomas in Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995 (eds Ries, L. A. G. et al.) 111–124 (National Cancer Institute, SEER Program. NIH Pub. No. 99–4649, Bethesda, MD, 1999).
Perez, E. A. et al. Rhabdomyosarcoma in children: a SEER population based study. J. Surg. Res. 170, 243–251 (2011).
Ferrari, A. et al. Paratesticular rhabdomyosarcoma: report from the Italian and German Cooperative Group. J. Clin. Oncol. 20, 449–455 (2002).
Raney, R. B. et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J. Clin. Oncol. 29, 1312–1318 (2011).
Rodeberg, D. A. et al. Comparison of outcomes based on treatment algorithms for rhabdomyosarcoma of the bladder/prostate: combined results from the Children's Oncology Group, German Cooperative Soft Tissue Sarcoma Study, Italian Cooperative Group, and International Society of Pediatric Oncology Malignant Mesenchymal Tumors Committee. Int. J. Cancer 128, 1232–1239 (2011).
Arndt, C. A. et al. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer 91, 2454–2468 (2001).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Punyko, J. A. et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: a report from the childhood cancer survivor study. Pediatr. Blood Cancer 44, 643–653 (2005).
Sung, L. et al. Late events occurring five years or more after successful therapy for childhood rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Eur. J. Cancer 40, 1878–1885 (2004).
Bassal, M. et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 24, 476–483 (2006).
Wallace, W. H. B., Anderson, R. A. A. & Irvine, D. S. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 6, 209–218 (2005).
Spunt, S. L. et al. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J. Clin. Oncol. 23, 7143–7151 (2005).
Mosiello, G. et al. Neurovesical dysfunction in children after treating pelvic neoplasms. BJU Int. 92, 289–292 (2003).
Agarwala, S. et al. Transitional cell carcinoma of the urinary bladder following exposure to cyclophosphamide in childhood. Eur. J. Pediatr. Surg. 11, 207–210 (2001).
Jerkins, G. R., Noe, H. N. & Hill, D. Treatment of complications of cyclophosphamide cystitis. J. Urol. 139, 923–925 (1988).
Arndt, C. et al. Does bladder preservation (as a surgical principle) lead to retaining bladder function in bladder/prostate rhabdomyosarcoma? Results from intergroup rhabdomyosarcoma study iv. J. Urol. 171, 2396–2403 (2004).
Yeung, C. K., Ward, H. C., Ransley, P. G., Duffy, P. G. & Pritchard, J. Bladder and kidney function after cure of pelvic rhabdomyosarcoma in childhood. Br. J. Cancer 70, 1000–1003 (1994).
Bernstein, L., Smith, M. A., Liu, L., Deapen, D. & Friedman, D. L. Germ Cell, Trophoblastic and Other Gonadal Neoplasms in Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995 (eds Ries, L. A. G. et al.) 125–138 (National Cancer Institute, SEER Program. NIH Pub. No. 99–4649 Bethesda, MD, 1999).
Schlatter, M. et al. Excellent outcomes of patients with stage I germ cell tumors of the testes: a study of the Children's Cancer Group/Pediatric Oncology Group. J. Pediatr. Surg. 38, 319–324 (2003).
Rogers, P. C. et al. Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study--Pediatric Oncology Group 9048 and Children's Cancer Group 8891. J. Clin. Oncol. 22, 3563–3569 (2004).
Cushing, B. et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study--Pediatric Oncology Group 9049 and Children's Cancer Group 8882. J. Clin. Oncol. 22, 2691–2700 (2004).
Pohl, H. G. et al. Prepubertal testis tumors: actual prevalence rate of histological types. J. Urol. 172, 2370–2372 (2004).
Patel, A. S., Coley, B. D. & Jayanthi, V. R. Ultrasonography underestimates the volume of normal parenchyma in benign testicular masses. J. Urol. 178, 1730–1732 (2007).
Goldiner, P. L., Carlon, G. C., Cvitkovic, E., Schweizer, O. & Howland, W. S. Factors influencing postoperative morbidity and mortality in patients treated with bleomycin. Br. Med. J. 1, 1664–1667 (1978).
Pui, C. H. et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N. Engl. J. Med. 12, 1682–1687 (1991).
Chabaud-Williamson, M. et al. Ovarian-sparing surgery for ovarian teratoma in children. Pediatr. Blood Cancer 57, 429–434 (2011).
Kreuser, E. D. et al. Reproductive and endocrine gonadal functions in adults following multidrug chemotherapy for acute lymphoblastic or undifferentiated leukemia. J. Clin. Oncol. 6, 588–595 (1988).
Bath, L. E., Wallace, W. H. & Critchley, H. O. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG 109, 107–114 (2002).
Williams, D. H. et al. Pretreatment semen parameters in men with cancer. J. Urol. 181, 736–740 (2009).
Edge, B., Holmes, D. & Makin, G. Sperm banking in adolescent cancer patients. Arch. Dis. Child. 91, 149–152 (2006).
Ginsberg, J. P. et al. Experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum. Reprod. 25, 37–41 (2010).
Fabbri, R. et al. Cryopreservation of ovarian tissue in pediatric patients. Obstet. Gynecol. Int. 2012, 1–8 (2012).
Gracia, C. R. et al. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J. Assist. Reprod. Genet. 29, 495–502 (2012).
Ginsberg, J. P. The effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur. J. Pediatr. 170, 703–708 (2011).
Kemp, G. et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J. Clin. Oncol. 14, 2101–2112 (1996).
Marina, N. et al. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children's Oncology Group study. Cancer 104, 841–847 (2005).
Ginsberg, S. J. & Comis, R. L. The pulmonary toxicity of antineoplastic agents. Semin. Oncol. 9, 34–51 (1982).
Mertens, A. C. et al. Pulmonary complications in survivors of childhood and adolescent cancer: a report from the Childhood Cancer Survivor Study. Cancer 95, 2431–2441 (2002).
Mertens, A. et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J. Clin. Oncol. 19, 3163–3172 (2001).
Stohr, W. et al. Nephrotoxicity of cisplatin and carboplatin in sarcoma patients: a report from the late effects surveillance system. Pediatr. Blood Cancer 48, 140–147 (2007).
Liao, F., Folsom, A. R. & Brancati, F. L. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 136, 480–490 (1998).
Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers [online], (2012).
Author information
Authors and Affiliations
Contributions
K. T. Sadak and J. S. Dome made substantial contributions to discussions of content and reviewed and edited the manuscript before submission. All authors researched data for the article and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sadak, K., Ritchey, M. & Dome, J. Paediatric genitourinary cancers and late effects of treatment. Nat Rev Urol 10, 15–25 (2013). https://doi.org/10.1038/nrurol.2012.218
Published:
Issue date:
DOI: https://doi.org/10.1038/nrurol.2012.218
This article is cited by
-
High-risk blastemal Wilms tumor can be modeled by 3D spheroid cultures in vitro
Oncogene (2020)
-
Discrepant outcomes in two Brazilian patients with Bloom syndrome and Wilms’ tumor: two case reports
Journal of Medical Case Reports (2013)