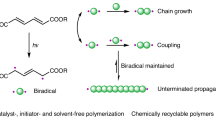
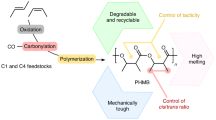
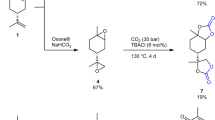
Abstract
Recent insight into the mechanism of carbocationic polymerizations have led to significant advances toward the synthesis of many types of unique well-defined macromolecules. Elucidation of details of initiation yielded polyisobutylylstyrene macromer CH2=CH–C6H4–PIB, the free-radical solution copolymerization of which with various acrylates led to new grafts, e.g., poly(butyl acrylate-g-isobutylene). The inifer technique led to α,ω)-di(t-chloro)polyisobutylene which was converted in 100% yield to α,ω)-bis(2-methyl-2-propenyl)polyisobutylene which upon hydroboration and alkaline oxidation resulted in 100% yield in α,ω-bis(3-hydroxyl-2-methylpropyl)polyisobutylene, HOCH2–PIB-CH2OH. Extensive characterization research of the latter α,ω-primary diol indicated Fn (average terminal functionality)=2.0. This α,ω-primary diol in conjunction with 2,4-toluene diisocyanate gave new polyurethanes with polyisobutylene soft segments. On account of their saturated hydrocarbon soft-segments these polyurethanes promise to exhibit improved UV, chemical and hydrolytic stability. Analysis of kinetic factors that control the rates of termination, chain transfer to monomer, initiation and propagation, Rt, Rtr,M, Ri, Rp, led to the prediction and subsequent experimental demonstration of quasiliving carbocationic polymerizations. Under quasiliving conditions the system behaves as though Rt=0, Rtr,M=0, and Ri>Rp. Quasiliving polymerizations have been obtained with α-methylstyrene and isobutylene monomers. The molecular weights of polymers obtained under quasiliving conditions obey D̅P̅n=Mtotal/[I]0, where Mtotal is monomer input and [I]0 is initial initiator concentration. Increased understanding of termination processes led to polymers with desirable terminal functionalities, e.g., chlorine, cyclopentadiene, and phenyl. The controlled combination of mechanistic elements, i.e., controlled initiation, propagation (absence of chain transfer to monomer) and termination has resulted in the synthesis of the first graft-block copolymer, poly[chloroprene-g-(isobutylene-b-α-methylstyrene)] by carbocationic techniques.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
“Cationic Graft Copolymerization,” J. P. Kennedy, Ed., J. Appl. Polym. Sci., Appl. Polym. Symp., 30, 1—192 (1977).
F. Denes, V. Percec, M. Totolin, and J. P. Kennedy, Polym. Bull., in press.
A. Vidal, A. Guyot, and J. P. Kennedy, Polym. Bull., in press.
J. P. Kennedy and K. Frisch, unpublished results (Akron, 1979).
J. P. Kennedy and R. Chou, Polym. Prepr., Am. Chem. Soc., Div. Polym. Chem., 20, 306 (1979).
J. P. Kennedy and R. A. Smith, Polym. Prepr., Am. Chem. Soc., Div. Polym. Chem., 20, 316 (1979).
J. P. Kennedy and R. A. Smith, J. Polym. Sci., Polym. Chem. Ed., in press.
A. Fehérvári, J. P. Kennedy, and F. Tüdös, Poym. Prepr., Am. Chem. Soc., Div. Polym. Chem., 20, 320 (1978).
B. Iván, J. P. Kennedy, T. Kelen, and F. Tüdös, J. Macromol. Sci., Chem., in press.
A. Fehérvári, J. P. Kennedy, and F. Tüdös, J. Macromol. Sci., Chem., in press.
J. P. Kennedy and R. A. Smith, J. Polym. Sci., Polym. Chem. Ed., in press.
J. P. Kennedy, V.S.-C. Chang, R. A. Smith, and B. Ivan, Polym. Bull., 1, 575 (1979).
B. Iván, J. P. Kennedy, and V.S.-G. Chang, J. Polym. Sci., Polym. Chem. Ed., in press.
B. Iván and J. P. Kennedy, Polym. Prepr., Am. Chem. Soc., Div. Polym. Chem., in press.
M. Szwarc, Nature, 178, 1168 (1956).
M. Szwarc, M. Levy, and R. Milkovich, J. Am. Chem. Soc., 78, 2656 (1956).
J. P. Kennedy, E. Melby, and J. E. Johnston, J. Macromol. Sci., Chem., A8, 463 (1974).
T. Higashimura, O. Kishihiro, and T. Takeda, J. Polym. Sci., Polym. Chem. Ed., 14, 1089 (1976).
T. Higashimura and O. Kishihiro, Polym. J., 9, 87 (1977).
T. Higashimura, M. Mitsuhashi, and M. Sawamoto, Macromolecules, 12, 178 (1979).
D. C. Pepper, J. Polym. Sci., Polym. Symp., No. 50, 51 (1975).
R. Faust, A. Fehérvári, and J. P. Kennedy, to be published (1980).
J. P. Kennedy, Makromol. Chem., Suppl., 3, 1 (1979).
J. P. Kennedy and S. S. Plamthottam, to be published (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kennedy, J. Tailor-Made Macromolecules by Carbocationic Techniques. Polym J 12, 609–615 (1980). https://doi.org/10.1295/polymj.12.609
Issue date:
DOI: https://doi.org/10.1295/polymj.12.609