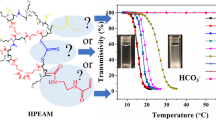
Abstract
To obtain a temperature-responsive CO2 absorbent with a high amine content, temperature-responsive gel particles (GPs) consisting of poly(N-isopropylacrylamide-co-polyvinylamine) (poly(NIPAm-co-VAm)) were designed and prepared. Stable poly(N-isopropylacrylamide) GPs with a high N-vinylformamide (NVF) content were prepared in aqueous media by optimization of the concentrations of the surfactant and monomers used in the polymerization step. GPs with a high polyvinylamine (pVAm) content up to 3.2 mmol amine per g GPs (~30 mol%) were prepared as a stable aqueous solution via the complete and selective hydrolysis of pNVF in the poly(NIPAm-co-NVF) GPs in a methanol solution, then dialyzed against water. The GPs with 3.2 mmol amine per g GPs pVAm showed a volume phase transition at a temperature of ~65 °C. Conductivity experiments established that the aqueous solution of the poly(NIPAm-co-VAm) GPs reversibly absorbed CO2 in response to a small thermal stimulus (30–75 °C). In addition, the foaming of amine-functionalized GP solutions during CO2 bubbling was prevented by increasing the amount of crosslinking in the GPs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Solomon, S., Qin, D., Manning., M., Marquis, M., Averyt, K., Tignor, M. M. B. Jr, Miller, H. L. & Chen, Z. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, Cambridge, 2007).
Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652 (2009).
Chowdhury, F. A., Okabe, H., Yamada, H., Onoda, M. & Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 4, 201 (2011).
Matsuzaki, Y., Yamada, H., Chowdhury, F. A., Higashii, T., Kazama, S. & Onoda, M. Ab initio study of CO2 capture mechanisms in monoethanolamine aqueous solution: reaction pathways from carbamate to bicarbonate. Energy Procedia 37, 400 (2013).
Murai, S., Kato, Y., Maezawa, Y., Muramatsu, T. & Saito, S. Novel hindered amine absorbent for CO2 capture. Energy Procedia 37, 417 (2013).
Goeppert, A., Czaun, M., May, R. B., Prakash, G. K. S., Olah, G. A. & Narayanan, S. R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 133, 20164 (2011).
Keskin, S., van Heest, T. M. & Sholl, D. S. Can metal–organic framework materials play a useful role in large-scale carbon dioxide separations? ChemSusChem 3, 879 (2010).
Furusho, Y. & Endo, T. Capture and release of CO2 by polyamidine. J. Polym. Sci. A Polym. Chem. 51, 3404 (2013).
Ochiai, B., Yokota, K., Fujii, A., Nagai, D. & Endo, T. Reversible trap−release of CO2 by polymers bearing DBU and DBN moieties. Macromolecules 41, 1229 (2008).
Trewin, A. & Cooper, A. I. Porous organic polymers: distinction from disorder? Angew. Chem. Int. Ed. 49, 1533 (2010).
Lu, W., Sculley, J. P., Yuan, D., Krishna, R., Wei, Z. & Zhou, H. C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 51, 7480 (2012).
Choi, S., Drese, J. H. & Jones, C. W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796 (2009).
D'Alessandro, D. M., Smit, B. & Long, J. R. Carbon dioxide capture: prospects for new materials. Angew. Chem., Int. Ed. 49, 6058 (2010).
Hoshino, Y., Imamura, K., Yue, M., Inoue, G. & Miura, Y. Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J. Am. Chem. Soc. 134, 18177 (2012).
Yue, M., Hoshino, Y., Ohshiro, Y., Imamura, K. & Miura, Y. Temperature-responsive microgel films as reversible carbon dioxide absorbents in wet environment. Angew. Chem. Int. Ed. 53, 2654 (2014).
Yue, M., Hoshino, Y. & Miura, Y. Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles. Chem. Sci. 6, 6112 (2015).
Werz, P. D. L., Kainz, J. & Rieger, B. Thermo- and pH-responsive nanogel particles bearing secondary amine functionalities for reversible carbon dioxide capture and release. Macromolecules 48, 6433 (2015).
Pelton, R. H. & Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloid Surf. 20, 247 (1986).
Fujishig, S., Kubota, K. & Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 93, 3311 (1989).
Akashi, M., Nakano, S. & Kishida, A. Synthesis of poly(N-vinylisobutyramide) from poly(N-vinylacetamide) and its thermosensitive property. J. Polym. Sci. A Polym. Chem. 34, 301 (1996).
Tachaboonyakiat, W., Ajiro, H. & Akashi, M. Synthesis of a thermosensitive polycation by random copolymerization of N-vinylformamide and N-vinylbutyramide. Polym. J. 45, 971 (2013).
Yamamoto, K., Serizawa, T., Muraoka, Y. & Akashi, M. Synthesis and functionalities of poly(N-vinylalkylamide). 13. Synthesis and properties of thermal and pH stimuli-responsive poly(vinylamine) copolymers. Macromolecules 34, 8014 (2001).
Takemoto, Y., Ajiro, H., Asoh, T.-A. & Akashi, M. Fabrication of surface-modified hydrogels with polyion complex for controlled release. Chem. Mater. 22, 2923 (2010).
Xu, J., Timmons, A. B. & Pelton, R. H. N-Vinylformamide as a route to amine-containing latexes and microgels. Colloid. Polym. Sci. 282, 256 (2004).
Fineman, M. & Ross, S. D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 5, 259 (1950).
Kelen, T. & Tudos, F. J. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J. Macromol. Sci. Chem. 9, 1 (1975).
Thaiboonrod, S., Berkland, C., Milani, A. H., Ulijn, R. & Saunders, B. R. Poly(vinylamine) microgels: pH-responsive particles with high primary amine contents. Soft Matter 9, 3920 (2013).
Abe, S. & Yamaguchi, M. Study on the foaming of crosslinked polyethylene. J. Appl. Polym. Sci. 79, 2146 (2001).
Yue, M., Imai, K., Yamashita, C., Miura, Y. & Hoshino, Y. Effects of hydrophobic modifications and phase transitions of polyvinylamine hydrogel films on reversible CO2 capture behavior: comparison between copolymer films and blend films for temperature-responsive CO2 absorption. Macromol. Chem. Phys. 218, 1600570 (2017).
Winnik, F. M., Ringadorf, H. & Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 23, 2415 (1990).
Vaidya, P. D. & Kenig, E. Y. CO2-alkanolamine reaction kinetics: a review of recent studies. Chem. Eng. Technol. 30, 1467 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yue, M., Imai, K., Miura, Y. et al. Design and preparation of thermo-responsive vinylamine-containing micro-gel particles for reversible absorption of carbon dioxide. Polym J 49, 601–606 (2017). https://doi.org/10.1038/pj.2017.28
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2017.28
This article is cited by
-
Hydrogel particles for CO2 capture
Polymer Journal (2024)
-
Adsorption of Pb(II) ion by pyromellitic dianhydride-modified polyvinylamine under programmed-temperature
Chemical Papers (2023)
-
Frontiers of CO2 Capture and Utilization (CCU) towards Carbon Neutrality
Advances in Atmospheric Sciences (2022)
-
Synthesis and characterization of cryogels of p(HEMA-N-vinylformamide) and p(HEMA-N-Vinylpyrrolidone) for chemical release behaviour
Journal of Porous Materials (2021)
-
Preparation of palladium-loaded polymer hydrogel catalysts with high durability and recyclability
Polymer Journal (2020)