Abstract
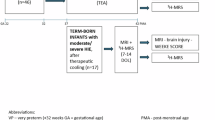
Proton magnetic resonance spectroscopy (1H MRS) was performed within 18 h of birth (median 13, range 4-18 h) on 16 term infants with clinical features of birth asphyxia. Ten infants with no evidence of birth asphyxia were studied as controls at 5-18 (median 8) h after birth. To detect delayed impairments in cerebral energy metabolism, 15 infants suspected of asphyxia underwent 31P MRS at 33-106 (median 62) h of age. Choline, creatine, and N-acetylaspartate (NAA) were detected in spectra located to the basal ganglia in all infants. Lactate was detected in 15 of the 16 infants suspected of asphyxia, but in only 4 of the 10 controls (p < 0.05, χ2). Glutamine and glutamate (Glx) was detected in 11 infants suspected of asphyxia and in three controls, but this difference was not significant at the 5% level. The spectra revealed no other significant differences between asphyxiated infants and controls. In the asphyxiated infants, there was a negative correlation between the ratio of lactate to creatine in the first 18 h of life and phosphocreatine/inorganic phosphate(PCr/Pi) at 33-106 h (p < 0.001). Five severely asphyxiated infants had PCr/Pi < 0.75 (median 0.53, range 0.14-0.65), indicating a poor neurodevelopmental prognosis, and a further infant died before PCr/Pi could be measured. Ten infants had PCr/Pi > 0.75 (1.03, 0.76-1.49). Median lactate/creatine was 1.47(range 0.67-3.81) in the six severely affected subjects, 0.38 (0-1.51) in the latter group, and 0 (0-0.6) in controls (p < 0.0005, Kruskall-Wallis). These results suggest that, after birth asphyxia, cerebral energy metabolism is abnormal during the period when 31P MRS characteristically gives normal results. 1H MRS might be of value in predicting which infants are likely to suffer a decline in cerebral high energy phosphate concentrations and subsequent neurodevelopmental impairment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 1H:
-
proton
- MRS:
-
magnetic resonance spectroscopy
- NAA:
-
N-acetylaspartate
- Glx:
-
glutamine and glutamate
- PCr:
-
phosphocreatine
- Pi:
-
inorganic phosphate
- TE:
-
echo time
References
Alberman E 1982 The epidemiology of congenital defects: a pragmatic approach. In: Adinolfi M, Benson P, Giannelli A, Seller M (eds) Pediatric Research: A Genetic Approach. Heinemann, London, 1–12.
Hope PL, Costello AM, Cady EB, Delpy DT, Tofts PS, Chu A, Hamilton PA, Reynolds EOR, 1984 Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 2: 366–369.
Azzopardi D, Wyatt JS, Cady EB, Delpy DT, Baudin J, Stewart AL, Hope PL, Hamilton PA, Reynolds EOR 1989 Prognosis of newborn infants with hypoxicischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res 25: 445–451.
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Cooper CE, Aldridge RF, Roth SC, Brown G, Delpy DT, Reynolds EOR 1994 Delayed(secondary) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36: 699–706.
Blumberg R, Cady EB, Edwards DT 1995 Cerebral energy metabolism following transient hypoxia-ischaemia in the 14-day old rat, measured by 31P magnetic resonance spectroscopy. Early Hum Dev (in press)
Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D, Baudin J, Townsend J, Stewart AL, Reynolds EOR 1992 Relation between cerebral oxidative metabolism following birth asphyxia and neurodevelopmental outcome and brain growth at one year. Dev Med Child Neurol 34: 285–295.
Moorcroft J, Bolas NM, Ives NK, Ouwerkerk R, Smyth J, Rajagopalan B, Hope PL, Radda GK 1991 Global and depth resolved phosphorus magnetic resonance spectroscopy to predict outcome after birth asphyxia. Arch Dis Child 66: 1119–1123.
Peden CJ, Cowan FM, Bryant DJ, Sargentoni J, Cox IJ, Menon DK, Gadian DG, Bell JD, Dubowitz LM 1990 Proton MR spectroscopy of the brain in infants. J Comput Assisted Tomogr 14: 886–894.
Peden CJ, Rutherford MA, Sargentoni J, Cox IJ, Bryant DJ, Dubowitz LMS 1993 Proton spectroscopy of the neonatal brain following hypoxic-ischemic injury. Dev Med Child Neurol 35: 502–510.
Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS 1994 Cerebral lactate andN-acetylaspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res 35: 148–151.
Cady EB, Lorek A, Penrice J, Reynolds EOR, Iles RA, Burns SP, Coutts GA, Cowan FM 1994 Detection of propan-1,2-diol in neonatal brain by in vivo proton magnetic resonance spectroscopy. Magn Reson Med (in press)
Saeed N 1993 A knowledge based approach to suppress water component in in vivo proton spectroscopy. Proceedings of Society of Magnetic Resonance in Medicine, New York, p 403
Azzopardi D, Wyatt J, Hamilton PA, Cady EB, Delpy DT, Hope PL, Reynolds EOR 1989 Phosphorus metabolites and intracellular pH in the brains of normal and small for gestational age infants investigated by magnetic resonance spectroscopy. Pediatr Res 25: 440–444.
Sarnat HB, Sarnat MS 1976 Neonatal encephalopathy following fetal distress-a clinical and electroencephalographic study. Arch Neurol 33: 696–705.
Cady EB 1995 Quantitative combined phosphorus and proton PRESS of the brains of newborn human infants. Magn Res Med 33: 557–563.
Barkovich AJ 1992 MR and CT evaluation of profound neonatal and infantile asphyxia. Am J Neuroradiol 13: 959–972.
Rutherford MA, Pennock JM, Dubowitz LMS 1994 Cranial ultrasound and magnetic resonance imaging in hypoxic-ischemic encephalopathy: a comparison with outcome. Dev Med Child Neurol 36: 813–825.
Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N 1991 Magnetic resonance in preterm and term infants: 1H spectroscopy in developing human brain. Pediatr Res 30: 574–578.
van Rijen PC, Verheul HB, van Echteld CJA, Balazs R, Lewis P, Nasim MM, Tulleken CAF 1991 Effects of dextromethorphan on rat brain during ischemia and reperfusion assessed by magnetic resonance spectroscopy. Stroke 22: 343–350.
van der Knapp MS, van der Grond J, van Rijen PC, Faber JAJ, Valk J, Willemse K 1990 Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 176: 509–515.
Pryds O, Griesen G, Lou H, Friis-Hansen 1990 Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr 117: 119–125.
Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, Reynolds EOR 1990 Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol 68: 1086–1091.
Young RS, Chen B, Petroff OAC, Gore JC, Cowan BE, Novotny EJ, Wong M, Zuckerman K 1989 The effect of diazepam on neonatal seizure: in vivo31P and 1H NMR study. Pediatr Res 25: 27–31.
Asano H, Homan J, Carmichael L, Richardson B 1994 Cerebral metabolism during sustained hypoxemia in the preterm fetal sheep. Am J Obstet Gynecol 170: 939–944.
De Haan HH, Ijzermans ACM, de Haan J, van Belle H, Hasaart TMH 1994 Effects of surgery and asphyxia on levels of nucleosides, purine bases and lactate in cerebrospinal fluid of fetal lambs. Pediatr Res 36: 595–600.
Young RS, Petroff OA, Chen B, Aquila WJ, Gore JC 1991 Preferential utilisation of lactate in neonatal dog brain: in vivo and in vitro proton NMR study. Biol Neonate 59: 46–53.
Gideon P, Henrikson O, Sperling B, Christiansen P, Olsen TS, Jorgensen HS, Arlien-Soborg P 1992 Early time course ofN-acetylaspartate, creatine and phosphocreatine, and compounds containing choline in the brain after acute stroke. Stroke 23: 1566–1572.
Graham GD, Blamire AM, Houseman AM, Rothman DL, Fayad PB, Brass LM, Petroff OAC, Shulman RG, Prichard JW 1992 Proton magnetic resonance spectroscopy of cerebral lactate and other metabolites in stroke patients. Stroke 23: 333–340.
Rothman DL, Houseman AM, Graham GD, Petroff OAC, Lantos G, Fayad PB, Brass LM, Shulman GI, Shulman RG, Prichard JW 1991 Localized proton NMR observation of [3-13C]lactate in stroke after[1-13C]glucose infusion. Magn Reson Med 21: 302–307.
Acknowledgements
The authors gratefully acknowledge the assistance of the staff of the Neonatal Units of Hammersmith and Queen Charlotte's Hospital.
Author information
Authors and Affiliations
Additional information
Supported by the Medical Research Council, Picker International, and the Garfield Weston Foundation.
Rights and permissions
About this article
Cite this article
Hanrahan, J., Sargentoni, J., Azzopardi, D. et al. Cerebral Metabolism within 18 Hours of Birth Asphyxia: A Proton Magnetic Resonance Spectroscopy Study. Pediatr Res 39, 584–590 (1996). https://doi.org/10.1203/00006450-199604000-00004
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199604000-00004
This article is cited by
-
MR spectroscopy in children: protocols and pitfalls in non-tumorous brain pathology
Pediatric Radiology (2016)
-
Correlations between maternal and neonatal serum selenium levels in full term neonates with hypoxic ischemic encephalopathy
Italian Journal of Pediatrics (2015)
-
Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic–ischemic encephalopathy
Pediatric Research (2013)
-
The effect of whole-body cooling on brain metabolism following perinatal hypoxic–ischemic injury
Pediatric Research (2012)
-
The prognostic value of multivoxel magnetic resonance spectroscopy determined metabolite levels in white and grey matter brain tissue for adverse outcome in term newborns following perinatal asphyxia
European Radiology (2012)