Abstract
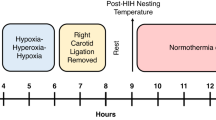
Platelet-activating factor (PAF) is overproduced in ischemic brain. Although postischemic PAF antagonist administration protects the mature brain in some models, little is known about the effects of PAF antagonists in the immature brain. We hypothesized that the PAF antagonist BN 52021 would attenuate perinatal cerebral hypoxic-ischemic injury. To elicit focal hypoxic-ischemic brain injury, 7-d-old (P7) rats (n = 111) underwent right carotid ligation, followed by 2.5-3.25 h of hypoxia (fractional concentration of inspired O2 = 0.08). BN 52021 neuroprotection was evaluated in three groups of experiments: 1) 25 mg/kg/dose, 0 and 2 h posthypoxia; 2), 25 mg/kg/dose immediately before and 1 h after hypoxia; and 3) posthypoxia-ischemia treatment with BN 52021 12.5, 25, or 50 mg/kg/dose in 2 doses 0 and 2 h after hypoxia. All experiments included concurrent vehicle-injected controls. To quantitate severity of injury, bilateral regional cross-sectional areas (groups 1 and 2) or hemisphere weights (group 3) were evaluated on P12. Both preand posthypoxic treatment with BN 52021 (25 mg/kg/dose, two serial doses) decreased the incidence of cerebral infarction from 90% to about 30% (p < 0.02, Fisher's exact test). Measurement of cross-sectional areas confirmed neuroprotection and indicated some benefit of pre- over posthypoxic-ischemic treatment in hippocampus and cortex. Over the dose range tested, the neuroprotective effect of BN 52021 administration was not dose-dependent. In contrast, BN 52021 did not attenuate N-methyl-D-asparate-induced hippocampal excitotoxic injury in P7 rats. Either prophylactic or“rescue” administration of PAF antagonists decreases the incidence and severity of brain injury associated with an episode of perinatal cerebral hypoxia-ischemia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- EAA:
-
excitatory amino acid
- PAF:
-
platelet-activating factor
- P7, P8, P12:
-
postnatal d 7, 8, 12
- Fio2:
-
fractional concentration of inspired oxygen
- NMDA:
-
N-methyl-D-aspartate
- ANOVA:
-
analysis of variance
- MK-801:
-
(+)-5-methyl-10,11-dihydro-5H-dibenzo{a,d} cyclohepten-5-10-imine maleate
- NBQX:
-
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline
References
Barks JD, Silverstein FS 1992 Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathol 2: 235–243
Palmer C 1995 Hypoxic-ischemic encephalopathy: therapeutic approaches against microvascular injury, and role of neutrophils, PAF and free radicals. Clin Perinatol 22: 481–517
Hudome SM, Roberts RL, Housman C, Towfighi J, Palmer C 1995 Interaction of allopurinol and neutropenia in the reduction of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res 37: 380A
Ivacko JA, Sun R, Silverstein FS 1996 Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res 39: 39–47
McRae A, Gilland E, Bona E, Hagberg H 1995 Microglia activation after neonatal hypoxic-ischemia. Dev Brain Res 84: 245–252
Szaflarski J, Burtrum D, Silverstein FS 1995 Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke 26: 1093–1100
Hagberg H, Soder O, Gilland E, McRae A, Bona E, Blennow M 1995 Interleukin-1 and hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res 37: 380A( abstr)
Martin D, Chinookoswong N, Miller G 1994 The interleukin-1 receptor antagonist (rhIL-Ira) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol 130: 362–367
Doucet JP, Bazan NG 1992 Excitable membranes, lipid messengers, and immediate-early genes. Alteration of signal transduction in neuromodulation and neurotrauma. Mol Neurobiol 6: 407–424
Koltai M, Guinot P, Hosford D, Braquet PG 1994 Platelet-activating factor antagonists: scientific background and possible clinical applications. Adv Pharmacol 28: 81–167
Zablocka B, Lukasiuk K, Lazarewicz JW, Domanska-Janik K 1995 Modulation of ischemic signal by antagonists ofN- methyl-D-aspartate, nitric oxide synthase, and platelet-activating factor in gerbil hippocampus. J Neurosci Res 40: 233–240
Kunievsky B, Yavin E 1994 Production and metabolism of platelet-activating factor in the normal and ischemic fetal rat brain. J Neurochem 63: 2144–2151
Kumar R, Harvey SA, Kester M, Hanahan DJ, Olson MS 1988 Production and effects of platelet-activating factor in the rat brain. Biochim Biophys Acta 963: 375–383
Domingo MT, Spinnewyn B, Chabrier PE, Braquet P 1994 Changes in [H-3]PAF binding and PAF concentrations in gerbil brain after bilateral common carotid artery occlusion-a quantitative autoradiographic study. Brain Res 640: 268–276
Yue TL, Feuerstein GZ 1994 Platelet-activating factor: a putative neuromodulator and mediator in the pathophysiology of brain injury. Crit Rev Neurobiol 8: 11–24
Bito H, Honda Z, Nakamura M, Shimizu T 1994 Cloning, expression and tissue distribution of rat platelet-activating-factor-receptor cDNA. Eur J Biochem 221: 211–218
Bonavida B, Mencia-Huerta JM 1994 Platelet-activating factor and the cytokine network in inflammatory processes. Clin Rev Allergy 12: 381–395
Lindsberg PJ, Yue TL, Frerichs KU, Hallenbeck JM, Feuerstein G 1990 Evidence for platelet-activating factor as a novel mediator in experimental stroke in rabbits. Stroke 21: 1452–1457
Pettigrew LC, Meyer JJ, Craddock SD, Butler SM, Tai HH, Yokel RA 1995 Delayed elevation of platelet activating factor in ischemic hippocampus. Brain Res 691: 243–247
Kato K, Clark GD, Bazan NG, Zorumski CF 1994 Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature 367: 175–179
Izquierdo I, Fin C, Schmitz PK, Da Silva RC, Jerusalinsky D, Quillfeldt JA, Ferreira MBG, Medina JH, Bazan NG 1995 Memory enhancement by intrahippocampal, intraamygdala, or intraentorhinal infusion of platelet-activating factor measured in an inhibitory avoidance task. Proc Natl Acad Sci USA 92: 5047–5051
Clark GD, Happel LT, Zorumski CF, Bazan NG 1992 Enhancement of hippocampal excitatory synaptic transmission by platelet-activating factor. Neuron 9: 1211–1216
Zablocka B, Domanska-Janik K 1994 PAF antagonist, BN52021, inhibits [3H]-D-aspartate release after ischaemia in vitro. Neuroreport 6: 85–88
Prehn JHM, Krieglstein J 1993 Platelet-Activating factor antagonists reduce excitotoxic damage in cultured neurons from embryonic chick telencephalon and protect the rat hippocampus and neocortex from ischemic injury in vivo. J Neurosci Res 34: 179–188
Hofer RE, Christopherson TJ, Scheithauer BW, Milde JH, Lanier WL 1993 The effect of a platelet activating factor antagonist (BN 52021) on neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology 79: 347–353
Adachi H, Tsujimoto M, Hattori M, Arai H, Inoue K 1995 cDNA cloning of human cytosolic platelet-activating factor acetylhydrolase gamma-subunit and its mRNA expression in human tissues. Biochem Biophys Res Commun 214: 180–187
Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K 1994 Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature 370: 216–218
Marcheselli VL, Rossowska MJ, Domingo MT, Braquet P, Bazan NG 1990 Distinct platelet-activating factor binding sites in synaptic endings and in intracellular membranes of cerebral cortex. J Biol Chem 265: 9140–9145
Yager JY, Heitjan DF, Towfighi J, Vannucci RC 1992 Effect of insulin-induced and fasting hypoglycemia on perinatal hypoxic-ischemic brain damage. Pediatr Res 31: 138–142
Barks JD, Nair MP, Schwartz SA, Silverstein FS 1993 Potentiation of N-methyl-D-aspartate-mediated brain injury by a human immunodeficiency virus-1-derived peptide in perinatal rodents. Pediatr Res 34: 192–198
McDonald JW, Roeser NF, Silverstein FS, Johnston MV 1989 Quantitative assessment of neuroprotection against NMDA-induced brain injury. Exp Neurol 106: 289–296
Konigsmark BW 1970 Methods for the counting of neurons. In: Nauta WJH, Ebbesson SOE (eds) Contemporary Research Methods in Neuroanatomy. Springer, New York, pp 315–340
Vannucci RC, Lyons DT, Vasta F 1988 Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke 19: 245–250
Mujsce DJ, Christensen MA, Vannucci RC 1990 Cerebral blood flow and edema in perinatal hypoxic-ischemic brain damage. Pediatr Res 27: 450–453
Viswanath M, Palmer C, Roberts RL, Caplan MS 1996 Reduction of hypoxic ischemic brain injury in the neonatal rat with PAF antagonist WEB2170. Pediatr Res 39: 382A( abstr)
Lefer AM, Weyrich AS, Buerke M 1994 Role of selectins, a new family of adhesion molecules, in ischaemia-reperfusion injury. Cardiovasc Res 28: 289–294
Panetta T, Marcheselli VL, Braquet P, Spinnewyn B, Bazan NG 1987 Effects of a platelet activating factor antagonist (BN 52021) on free fatty acids, diacylglycerols, polyphosphoinositides and blood flow in the gerbil brain: inhibition of ischemia-reperfusion induced cerebral injury. Biochem Biophys Res Commun 149: 580–587
Bielenberg GW, Wagener G, Beck T 1992 Infarct reduction by the platelet activating factor antagonist apafant in rats. Stroke 23: 98–103
Nishida K, Markey SP 1996 Platelet-activating factor in brain regions after transient ischemia in gerbils. Stroke 27: 514–518
Feuerstein GZ 1996 Platelet-activating factor in brain regions after transient ischemia in gerbils [Editorial comment]. Stroke 27: 518–519
Ikonomidou C, Mosinger JL, Salles KS, Labruyere J, Olney JW 1989 Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-aspartate neurotoxicity. J Neurosci 9: 2809–2818
Szaflarski J, Liu XH, Warren JS, Silverstein FS 1995 Systemic administration of anti-monocyte chemoattractant protein-1 antibody attenuates excitotoxic brain injury in perinatal rodents. Soc Neurosci Abstr 21: 73
Hattori H, Morin AM, Schwartz PH, Fujikawa DG, Wasterlain CG 1989 Posthypoxic treatment with MK-801 reduces hypoxic-ischemic damage in the neonatal rat. Neurology 39: 713–718
Hagberg H, Gilland E, Diemer N-H, Andiné P 1994 Hypoxia-ischemia in the neonatal rat brain: Histopathology after post-treatment with NMDA and non-NMDA receptor antagonists. Biol Neonate 66: 205–213
Clark GD, McNeil RS, Bix GJ, Swann JW 1995 Platelet-activating factor produces neuronal growth cone collapse. Neuroreport 6: 2569–2575
Author information
Authors and Affiliations
Additional information
Supported by United Cerebral Palsy grant R-608-94 (to J.D.E.B.), American Heart Association Grant 92-845 (to F.S.S.), and U.S. Public Health Service Grant NS 31054 (to F.S.S. and J.D.E.B.).
Rights and permissions
About this article
Cite this article
Liu, XH., Eun, BL., Silverstein, F. et al. The Platelet-Activating Factor Antagonist BN 52021 Attenuates Hypoxic-Ischemic Brain Injury in the Immature Rat. Pediatr Res 40, 797–803 (1996). https://doi.org/10.1203/00006450-199612000-00004
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199612000-00004
This article is cited by
-
The effect of oxygen and light on the structure and function of the neonatal rat retina
Documenta Ophthalmologica (2009)
-
Hsp70 Overexpression Sequesters AIF and Reduces Neonatal Hypoxic/Ischemic Brain Injury
Journal of Cerebral Blood Flow & Metabolism (2005)