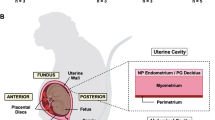
Abstract
The uterus is unique among smooth muscular organs in that, during pregnancy, it undergoes profound, largely reversible, changes orchestrated by the ovarian hormones. These changes facilitate uterine adaptation to the stretch induced by the growing fetus such that a state of myometrial contractile quiescence can be maintained. This quiescent state usually is maintained until fetal development is sufficient for extrauterine life, at which point unknown mechanisms precipitate conversion to a highly contractile state. Throughout pregnancy, signaling mechanisms for myometrial contractility are altered-first to promote quiescence and then again to promote contractions. The mechanisms responsible for these changes are only partially understood. This review attempts to summarize salient features of many of the changes in uterine contractile signaling and the current state of ongoing investigations of their mechanisms. We have also highlighted some newer information and concepts from nonuterine tissues, which we believe may provide insight into the control of uterine smooth muscle function. Some detail has been omitted, and can be found in the many excellent reviews cited. We hope that this discussion may stimulate the interests of other investigators. The diverse areas of inquiry offer hope that this decade will lead to a fuller understanding of myometrial function and the development of vastly improved approaches for the control of preterm labor.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- C43:
-
connexin 43
- CRH:
-
corticotrophin-releasing hormone
- DHEAS:
-
dehydroepiandrosterone sulfate
- EP1-4:
-
E-type prostaglandin receptors 1-4
- ET:
-
endothelin
- Gs, Gq:
-
G proteins sq
- IP3:
-
inositol 1,4,5-triphosphate
- IP3R:
-
inositol 1,4,5-triphosphate receptor
- KCa:
-
high conductance Ca2+-activated K+ channel
- MLC:
-
myosin light chain
- MLC-P:
-
myosin light chain phosphatase
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- OT:
-
oxytocin
- PG:
-
prostaglandin
- PGDH:
-
prostaglandin dehydrogenase
- PLC:
-
phospholipase C
- Ras:
-
small GTP-binding protein of 21 Ras superfamily
- Rac-1:
-
Rho
- RhoA:
-
subfamily members of Ras superfamily
- RyR:
-
ryanodine receptor, ryanodine-sensitive intracellular
- Ca2+:
-
channel
- SR:
-
sarcoplasmic reticulum
- VOC:
-
voltage-operated Ca2+ channels
References
Bulbring E, Tomita T 1987 Catecholamine action on smooth muscle. Pharmacol Rev 39: 49–96
Parkington HC, Harding R, Sigger JN 1988 Co-ordination of electrical activity in the myometrium of pregnant ewes. J Reprod Fertil 82: 697–705
Lammers WJEP, Arafat K, el-Kays A, el-Sharkawy TY 1994 Spatial and temporal variations in local spike propagation in the myometrium of the 17-day pregnant rat. Am J Physiol 267: C1210–C1223
Lammers WJ, Ahmad HR, Arafat K 1996 Spatial and temporal variations in pacemaking and conduction in the isolated renal pelvis. Am J Physiol 270: F567–F574
Word RA 1995 Myosin phosphorylation and the control of myometrial contraction/relaxation. Semin Perinatol 19: 3–14
Horowitz A, Menice CB, Laporte R, Morgan KG 1996 Mechanisms of smooth muscle contraction. Physiol Rev 76: 967–1003
Bárány K, Bárány M 1990 Myosin light chain phosphorylation in uterine smooth muscle. In: Carsten ME, Miller JD (eds) Uterine Functions: Molecular and Cellular Aspects. Plenum Press, New York, 71–98.
Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K 1996 Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248
Tapon N, Hall A 1997 Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol 9: 86–92
Ruzycky AL 1997 Pregnancy increases the expression of cytoskeletal signaling GTPases, rac-1 and RhoA, in human uterine myocytes. J Soc Gynecol Invest 4( Suppl): 162( abstr)
Ruzycky AL 1997 Agonist-specific changes in actin filament polymerization are associated with increased cytoskeletal signaling in the rat myometrium. J Soc Gynecol Invest 4( suppl): 106A( abstr)
Wray S 1993 Uterine contraction and physiological mechanisms of modulation. Am J Physiol 264: C1–C18
Fuchs AR 1995 Plasma, membrane receptors regulating myometrial contractility and their hormonal modulation. Semin Perinatol 19: 15–30
Szal SE, Repke JT, Seely EW, Graves SW, Parker CA, Morgan KG 1994 [Ca2+]i signaling in pregnant human myometrium. Am J Physiol 267: E77–E87
Sanborn BM 1995 Ion channels and the control of myometrial electrical activity. Semin Perinatol 19: 31–40
Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L 1993 mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science 261: 221–224
Bootman MD, Berridge MJ 1995 The elemental principles of calcium signaling. Cell 83: 675–678
Berridge MJ 1997 Elementary and global aspects of calcium signaling-annual review prize lecture. J Physiol 499: 291–306
Yue DT 1997 Quenching the spark in the heart. Science 276: 755–756
Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ 1995 Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637
Lynn S, Morgan JM, Lamb HK, Meissner G, Gillespie JI 1995 Isolation and partial cloning of ryanodine-sensitive Ca2+ release channel protein isoforms from human myometrial smooth muscle. FEBS Lett 372: 6–12
Gebremedhin D, Kaldunski M, Jacobs ER, Harder DR, Roman RJ 1996 Coexistence of two types of Ca2+-activated K+ channels in rat renal arterioles. Am J Physiol 270: F69–F81
Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L 1993 Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol 265: C976–C985
MacDonald PC, Casey ML 1993 The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 alpha in the initiation of human parturition. J Clin Endocrinol Metab 76: 1332–1339
Meera P, Anwer K, Monga M, Oberti C, Stefani E, Toro L, Sanborn BM 1995 Relaxin stimulates myometrial calcium-activated potassium channel activity via protein kinase A. Am J Physiol 269: C312–C317
Hamaguchi M, Ishibashi T, Imai S 1992 Involvement of charybdotoxin-sensitive K+ channel in the relaxation of bovine tracheal smooth muscle by glyceryl trinitrate and sodium nitroprusside. J Pharmacol Exp Ther 262: 263–270
Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA 1994 Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850–853
Dworetzky SI, Trojnacki JT, Gribkoff VK 1994 Cloning and expression of a human large-conductance calcium-activated potassium channel. Brain Res Mol Brain Res 27: 189–193
Khan RN, Smith SK, Morrison JJ, Ashford ML 1993 Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc R Soc Lond B Biol Sci 251: 9–15
Folander K, Smith JS, Antanavage J, Bennett C, Stein RB, Swanson R 1990 Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci USA 87: 2975–2979
Kasai Y, Tsutsumi O, Taketani Y, Endo M, Iino M 1995 Stretch-induced enhancement of contractions in uterine smooth muscle of rats. J Physiol 486: 373–384
Crozatier B 1996 Stretch-induced modifications of myocardial performance: from ventricular function to cellular and molecular mechanisms. Cardiovasc Res 32: 25–37
Arai A, Kodama I, Toyama J 1996 Roles of Cl- channels and Ca2+ mobilization in stretch-induced increase of SA node pacemaker activity. Am J Physiol 270: H1726–H1735
Hansen DE, Stacy GP J, Taylor LK, Jobe RL, Wang Z, Denton PK, Alexander J Jr 1995 Calcium- and sodium-dependent modulation of stretch-induced arrhythmias in isolated canine ventricles. Am J Physiol 268: H1803–H1813
Nakayama K, Tanaka Y 1993 Stretch-induced contraction and Ca2+ mobilization in vascular smooth muscle. Biol Signals 2: 241–252
Wilson E, Sudhir K, Ives HE 1995 Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest 96: 2364–2372
Bloom S, Lockard VG, Bloom M 1996 Intermediate filament-mediated stretch-induced changes in chromatin: a hypothesis for growth initiation in cardiac myocytes. J Mol Cell Cardiol 28: 2123–2127
Pommerenke H, Schreiber E, Durr F, Nebe B, Hahnel C, Moller W, Rychly J 1996 Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur J Cell Biol 70: 157–164
Wright M, Jobanputra P, Bavington C, Salter DM, Nuki G 1996 Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: evidence for the presence of stretch-activated membrane ion channels. Clin Sci 90: 61–71
Ingber D 1991 Integrins as mechanochemical transducers. Curr Opin Cell Biol 3: 841–848
Lab MJ 1996 Mechanoelectric feedback (transduction) in heart: concepts and implications. Cardiovasc Res 32: 3–14
Topper JN, Cai J, Falb D, Gimbrone MA Jr 1996 Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 93: 10417–10422
Black SM, Johengen M, Bristow J, Soifer SJ 1996 Ventilation and oxygenation induce endothelial nitric oxide synthase gene expression in the lungs of fetal lambs. Pediatr Res 39: 326A( abstr)
Vandenberg JI, Rees SA, Wright AR, Powell T 1996 Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc Res 32: 85–97
Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ 1997 Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276: 800–806
Beyer EC 1993 Gap junctions. Int Rev Cytol 137C: 1: 37
Finbow ME, Pitts JD 1993 Is the gap junction channel-the connexon-made of connexin or ductin? J Cell Sci 106: 463–471
Hall JE, Gourdie RG 1995 Spatial organization of cardiac gap junctions can affect access resistance. Microsc Res Tech 31: 446–451
Risek B, Klier FG, Phillips A, Hahn DW, Gilula NB 1995 Gap junction regulation in the uterus and ovaries of immature rats by estrogen and progesterone. J Cell Sci 108: 1017–1032
ten Velde I, de Jonge B, Verheijck EE, van Kempen MJ, Analbers L, Gros D, Jongsma HJ 1995 Spatial distribution of connexin 43, the major cardiac gap junction protein, visualizes the cellular network for impulse propagation from sinoatrial node to atrium. Circ Res 76: 802–811
Garfield RE (ed) 1990 Uterine Contractility: Mechanisms of Control. Serono Symposia, USA, Norwell, MA
Garfield RE, Ali M, Yallampalli C, Izumi H 1995 Role of gap junctions and nitric oxide in control of myometrial contractility. Semin Perinatol 19: 41–51
Chen ZQ, Lefebvre D, Bai XH, Reaume A, Rossant J, Lye SJ 1995 Identification of two regulatory elements within the promoter region of the mouse connexin 43 gene. J Biol Chem 270: 3863–3868
Yu W, Dahl G, Werner R 1994 The connexin 43 gene is responsive to oestrogen. Proc R Soc Lond B Biol Sci 255: 125–132
Risek B, Guthrie S, Kumar N, Gilula NB 1990 Modulation of gap junction transcript and protein expression during pregnancy in the rat. J Cell Biol 110: 269–282
Risek B, Gilula NB 1991 Spatiotemporal expression of three gap junction gene products involved in fetomaternal communication during rat pregnancy. Development 113: 165–181
Chow L, Lye SJ 1994 Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol 170: 788–795
Albrecht JL, Atal NS, Tadros PN, Orsino A, Lye SJ, Sadovsky Y, Beyer EC 1996 Rat uterine myometrium contains the gap junction protein connexin 45, which has a differing temporal expression pattern from connexin 43. Am J Obstet Gynecol 175: 853–858
Miyoshi H, Boyle MB, MacKay LB, Garfield RE 1996 Voltage-clamp studies of gap junctions between uterine muscle cells during term and preterm labor. Biophys J 71: 1324–1334
Andersen HF, Barclay ML 1995 A computer model of uterine contractions based on discrete contractile elements. Obstet Gynecol 86: 108–111
Young RC, Hession RO 1996 Intra-and intercellular calcium waves in cultured human myometrium. J Muscle Res Cell Motil 17: 349–355
Young RC 1997 A computer model of uterine contractions based on action potential propagation and intercellular calcium waves. J Soc Gynecol Invest 4: 160A( abstr)
Stjernquist M, Sjöberg N-O 1994 Neurotransmitters in the myometrium. In: Chard T, Grudzinskas JG (eds) The Uterus. University Press, Cambridge, 193–229.
Sjoberg NO 1968 Considerations on the cause of disappearance of the adrenergic transmitter in uterine nerves during pregnancy. Acta Physiol Scand 72: 510–517
Riemer RK, Buscher C, Bansal RK, Black SM, He Y, Natuzzi ES 1997 Increased expression of nitric oxide synthase in the myometrium of the pregnant rat uterus. Am J Physiol 272: E1008–E1015
Kawarabayashi T 1994 Electrophysiology of the human myometrium. In: Chard T, Grudzinskas JG (eds) The Uterus. University Press, Cambridge, 148–172.
Parkington HC, Coleman HA 1990 The role of membrane potential in the control of uterine motility. In: Carsten ME, Miller JD (eds) Uterine Function. Plenum, New York, 195–248.
Riemer RK, Goldfien AC, Goldfien A, Roberts JM 1986 Rabbit uterine oxytocin receptors and in vitro contractile response: abrupt changes at term and the role of eicosanoids. Endocrinology 119: 699–709
McCoshen JA, Tulloch HV, Johnson KA 1989 Umbilical cord is the major source of prostaglandin E2 in the gestational sac during term labor. Am J Obstet Gynecol 160: 973–978
Mitchell BF, Rogers K, Wong S 1993 The dynamics of prostaglandin metabolism in human fetal membranes and decidua around the time of parturition. J Clin Endocrinol Metab 77: 759–764
Germain AM, Smith J, Casey ML, MacDonald PC 1994 Human fetal membrane contribution to the prevention of parturition: uterotonin degradation. J Clin Endocrinol Metab 78: 463–70
Mitchell BF, Wong S 1995 Metabolism of oxytocin in human decidua, chorion, and placenta. J Clin Endocrinol Metab 80: 2729–2733
Sangha RK, Walton JC, Ensor CM, Tai HH, Challis JR 1994 Immunohistochemical localization, messenger ribonucleic acid abundance, and activity of 15-hydroxyprostaglandin dehydrogenase in placenta and fetal membranes during term and preterm labor. J Clin Endocrinol Metab 78: 982–989
Slater D, Berger L, Newton R, Moore G, Bennett P 1994 The relative abundance of type 1 to type 2 cyclo-oxygenase mRNA in human amnion at term. Biochem Biophys Res Commun 198: 304–308
Tezuka N, Ali M, Chwalisz K, Garfield RE 1995 Changes in transcripts encoding calcium channel subunits of rat myometrium during pregnancy. Am J Physiol 269: C1008–C1017
Inoue Y, Sperelakis N 1991 Gestational change in Na+ and Ca2+ channel current densities in rat myometrial smooth muscle cells. Am J Physiol 260: C658–C663
Felipe A, Knittle TJ, Doyle KL, Tamkun MM 1994 Primary structure and differential expression during development and pregnancy of a novel voltage-gated sodium channel in the mouse. J Biol Chem 269: 30125–30131
George AL, Knittle TJ, Tamkun MM 1992 Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus-evidence for a distinct gene family. Proc Natl Acad Sci USA 89: 4893–4897
Knittle TJ, Doyle KL, Tamkun MM 1996 Immunolocalization of the mNav2.3 Na+ channel in mouse heart: upregulation in myometrium during pregnancy. Am J Physiol 270: C688–C696
Felipe A, Knittle TJ, Doyle KL, Snyders DJ, Tamkun MM 1994 Differential expression of Isk mRNAs in mouse tissue during development and pregnancy. Am J Physiol 267: C700–C705
Coleman RA, Smith WL, Narumiya S 1994 International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229
Funk CD, Furci L, FitzGerald GA, Grygorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM 1993 Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem 268: 26767–26772
Breyer RM, Davis LS, Nian C, Redha R, Stillman B, Jacobson HR, Breyer MD 1996 Cloning and expression of the rabbit prostaglandin EP4 receptor. Am J Physiol 270: F485–F493
Sando T, Usui T, Tanaka I, Mori K, Sasaki Y, Fukuda Y, Namba T, Sugimoto Y, Ichikawa A, Narumiya S 1994 Molecular cloning and expression of rat prostaglandin E receptor EP2 subtype. Biochem Biophys Res Commun 200: 1329–1333
An S, Yang J, Xia M, Goetzl EJ 1993 Cloning and expression of the EP2 subtype of human receptors for prostaglandin E2 . Biochem Biophys Res Commun 197: 263–270
Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, Narumiya S 1995 In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol 116: 2828–2837
Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, Narumiya S 1993 Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 365: 166–170
Negishi M, Sugimoto Y, Namba T, Irie A, Narumiya S, Ichikawa A 1995 Signal transductions of three isoforms of mouse prostaglandin E receptor EP3 subtype. Adv Prostaglandin Thromboxane Leukotriene Res 23: 255–257
Riemer RK, Goldfien A, Roberts JM 1987 Estrogen increases adrenergic- but not cholinergic-mediated production of inositol phosphates in rabbit uterus. Mol Pharmacol 32: 663–668
Riemer RK, Wu YY, Bottari SP, Jacobs MM, Goldfien A, Roberts JM 1988 Estrogen reduces β-adrenoceptor-mediated cAMP production and the concentration of the guanyl nucleotide-regulatory protein, Gs, in rabbit myometrium. Mol Pharmacol 33: 389–395
Europe-Finner GN, Phaneuf S, Watson SP, Lopez Bernal A 1993 Identification and expression of G-proteins in human myometrium: up-regulation of Gαs in pregnancy. Endocrinology 132: 2484–2490
Cohen-Tannoudji J, Mhaouty S, Elwardy-Merezak J, Lecrivain JL, Robin MT, Legrand C, Maltier JP 1995 Regulation of myometrial Gi2, Gi3, and Gq expression during pregnancy. Effects of progesterone and estradiol. Biol Reprod 53: 55–64
Maggi M, Vannelli GB, Peri A, Brandi ML, Fantoni G, Giannini S, Torrisi C, Guardabasso V, Barni T, Toscano V 1991 Immunolocalization, binding, and biological activity of endothelin in rabbit uterus: effect of ovarian steroids. Am J Physiol 260: E292–E305
Kajihara T, Tomioka Y, Hata T, Ghazizadeh M, Asano G 1996 Synthesis of endothelin-1 in rat uterus during pregnancy. J Histochem Cytochem 44: 953–957
Yallampalli C, Garfield RE 1994 Uterine contractile responses to endothelin-1 and endothelin receptors are elevated during labor. Biol Reprod 51: 640–645
Heluy V, Germain G, Fournier T, Ferre F, Breuiller-Fouche M 1995 Endothelin ETA receptors mediate human uterine smooth muscle contraction. Eur J Pharmacol 285: 89–94
Maggi M, Vannelli GB, Fantoni G, Baldi E, Magini A, Peri A, Giannini S, Gloria L, Del Carlo P, Casparis D 1994 Endothelin in the human uterus during pregnancy. J Endocrinol 142: 385–396
Svane D, Larsson B, Alm P, Andersson KE, Forman A 1993 Endothelin-1: immunocytochemistry, localization of binding sites, and contractile effects in human utero placental smooth muscle. Am J Obstet Gynecol 168: 233–241
Csapo AI 1956 Progesterone block. Am J Anat 98: 273
Casey ML, MacDonald PC 1996 Transforming growth factor-beta inhibits progesterone-induced enkephalinase expression in human endometrial stromal cells. J Clin Endocrinol Metab 81: 4022–4027
Olson DM, Mijovic JE, Sadowsky DW 1995 Control of human parturition. Semin Perinatol 19: 52–63
Karalis K, Goodwin G, Majzoub JA 1996 Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 2: 556–560
McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R 1995 A placental clock controlling the length of human pregnancy. Nat Med 1: 460–463
Collins PL, Moore JJ, Idriss E, Kulp TM 1996 Human fetal membranes inhibit calcium L-channel activated uterine contractions. Am J Obstet Gynecol 175: 1173–1179
Collins PL, Idriss E, Moore JJ 1995 Fetal membranes inhibit prostaglandin but not oxytocin-induced uterine contractions. Am J Obstet Gynecol 172: 1216–1223
Sanborn BM, Qian A, Ku CY, Wen Y, Anwer K, Monga M, Singh SP 1995 Mechanisms regulating oxytocin receptor coupling to phospholipase C in rat and human myometrium. Adv Exp Med Biol 395: 469–479
Liu M, Simon MI 1996 Regulation by cAMP-dependent protein kinease of a G-protein-mediated phospholipase C. Nature 382: 83–87
Neer EJ 1995 Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80: 249–257.
Ruzycky AL, Crankshaw DJ 1988 Role of inositol phospholipid hydrolysis in the initiation of agonist-induced contractions of rat uterus: effects of domination by 17 beta-estradiol and progesterone. Can J Physiol Pharmacol 66: 10–17
Sladek SM, Magness RR, Conrad KP 1997 Nitric oxide and pregnancy. Am J Physiol 272: R441–R463
Bansal RK, Goldsmith PC, He Y, Zaloudek CJ, Ecker JL, Riemer RK 1997 A decline in myometrial nitric oxide synthase expression is associated with labor and delivery. J Clin Invest 99: 2502–2508
Buhimschi I, Ali M, Jain V, Chwalisz K, Garfield RE 1996 Differential regulation of nitric oxide in the rat uterus and cervix during pregnancy and labour. Hum Reprod 11: 1755–1766
Monga M, Creasy RK 1995 Pharmacologic management of preterm labor. Semin Perinatol 19: 84–96
Mizuki J, Tasaka K, Masumoto N, Kasahara K, Miyake A, Tanizawa O 1993 Magnesium sulfate inhibits oxytocin-induced calcium mobilization in human puerperal myometrial cells: possible involvement of intracellular free magnesium concentration. Am J Obstet Gynecol 169: 134–139
Goodwin TM, Paul R, Silver H, Spellacy W, Parsons M, Chez R, Hayashi R, Valenzuela G, Creasy GW, Merriman R 1994 The effect of the oxytocin antagonist atosiban on preterm uterine activity in the human. Am J Obstet Gynecol 170: 474–478
Goodwin TM, Valenzuela GJ, Silver H, Creasy G 1996 Dose ranging study of the oxytocin antagonist atosiban in the treatment of preterm labor. Atosiban Study Group. Obstet Gynecol 88: 331–336
Modanlou HD, Beharry K, Padilla G, Iriye B 1996 Combined effects of antenatal corticosteroids and surfactant supplementation on the outcome of very low birth weight infants. J Perinatol 16: 422–430
Clyman RI 1996 Ductus arteriosus: In: Gluckman PD, Heymann MA (eds) Pediatrics and Perinatology: The Scientific Basis. Edward Arnold, London, pp
Arslan A, Zingg HH 1996 Regulation of COX-2 gene expression in rat uterus in vivo and in vitro. Prostaglandins 52: 463–481
Fuentes A, Spaziani EP, O'Brien WF 1996 The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins 52: 261–267
McManus OB, Harris GH, Giangiacomo KM, Feigenbaum P, Reuben JP, Addy ME, Burka JF, Kaczorowski GJ, Garcia ML 1993 An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry 32: 6128–6133
Kimura T, Takemura M, Nomura S, Nobunaga T, Kubota Y, Inoue T, Hashimoto K, Kumazawa I, Ito Y, Ohashi K, Koyama M, Azuma C, Kitamura Y, Saji F 1996 Expression of oxytocin receptor in human pregnant myometrium. Endocrinology 137: 780–785
Chibbar R, Miller FD, Mitchell BF 1993 Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J Clin Invest 91: 185–192
Lefebvre DL, Farookhi R, Larcher A, Neculcea J, Zingg HH 1994 Uterine oxytocin gene expression. I. Induction during pseudopregnancy and the estrous cycle. Endocrinology 134: 2556–2561
Zingg HH, Rozen F, Chu K, Larcher A, Arslan A, Richard S, Lefebvre D 1995 Oxytocin and oxytocin receptor gene expression in the uterus. Recent Prog Horm Res 50: 255–273
Mitchell BF, Chibbar R 1995 Synthesis and metabolism of oxytocin in late gestation in human decidua. Adv Exp Med Biol 395: 365–380
Fang X, Wong S, Mitchell BF 1996 Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during the late gestation and at parturition. Endocrinology 137: 3213–3219
Casey ML, Brown CE, Peters M, MacDonald PC 1993 Endothelin levels in human amniotic fluid at mid-trimester and at term before and during spontaneous labor. J Clin Endocrinol Metab 76: 1647–1650
MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudgett J 1995 Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81: 641–650
Laubach VE, Shesely EG, Smithies O, Sherman PA 1995 Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA 92: 10688–10692
Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY 1995 Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375: 408–411
Cobb JP, Danner RL 1996 Nitric oxide and septic shock. JAMA 275: 1192–1196
Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM 1996 Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93: 11699–11704
Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O 1995 Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83: 483–492
Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O 1995 Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83: 473–82
Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J 1995 Cardiac malformation in neonatal mice lacking connexin 43. Science 267: 1831–1834
Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9: 2266–2278
Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162–11166
Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS 1996 Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 93: 12626–12630
Davis VL, Couse JF, Goulding EH, Power SG, Eddy EM, Korach KS 1994 Aberrant reproductive phenotypes evident in transgenic mice expressing the wild-type mouse estrogen receptor. Endocrinology 135: 379–386
Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138: 863–870
Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, Andrews WW 1995 The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obst Gynecol 173: 1231–1235
Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, Menard MK, Caritis SN, Thurnau GR, Bottoms SF 1998 The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol 178: 562–567
Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP 1998 Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 178: 546–550
Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, Das A, Thom E, Johnson F, McNellis D, Miodovnik M, Van Dorsten JP, Caritis SN, Thurnau GR, Bottoms SF 1998 The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health 88: 233–238
Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Caritis SN 1996 The preterm prediction study: fetal fibronectin, bacterial vaginosis, and peripartum infection. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol 87: 656–660
Author information
Authors and Affiliations
Additional information
Supported in part by National Institutes of Health Grant HD 32518.
Rights and permissions
About this article
Cite this article
Riemer, R., Heymann, M. Regulation of Uterine Smooth Muscle Function during Gestation. Pediatr Res 44, 615–627 (1998). https://doi.org/10.1203/00006450-199811000-00001
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199811000-00001
This article is cited by
-
Toxicological Impact of Bisphenol A on Females’ Reproductive System: Review Based on Experimental and Epidemiological Studies
Reproductive Sciences (2024)
-
Enhanced contractile response to thrombin in the pregnant rat myometrium
British Journal of Pharmacology (2000)