Abstract
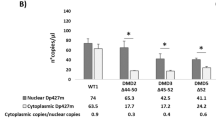
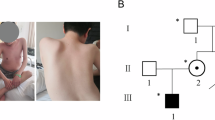
The molecular background of an intermediate type of dystrophinopathy [Duchenne and Becker muscular dystrophy (DMD/BMD)] remains to be clarified, and out-of -frame and in-frame mutations of the dystrophin gene are shown to be causes of DMD and BMD, respectively. In a boy with this disease, dystrophin mRNA extracted from lymphocytes and muscle were analyzed both qualitatively and quantitatively using reverse transcription PCR. Three different dystrophin mRNA were found to be produced via the use of three cryptic splice acceptor sites resulting from a novel point mutation of 2831-2A>G at the conserved splice acceptor site of intron 20. One of three mRNA showed an insertion of six nucleotides of intron 20 between exons 20 and 21 (dys+6) that encoded two novel amino acids in the rod domain of dystrophin. Two other mRNA species showed an insertion of seven nucleotides of intron 20 between exons 20 and 21 (dys+7) or a seven-nucleotide deletion in exon 21 (dys−7). Quantitative analysis of each dystrophin mRNA expressed in the boy's skeletal muscle disclosed that around 95% and 5% of dystrophin mRNAs were dys−7 and dys+6, respectively, whereas these two mRNA were almost equally expressed in lymphocytes. It is suggested that production of a small fraction of in-frame mRNA in muscle explains the molecular background of the intermediate type of dystrophinopathy in the index case. This finding underlines the potential of genetic therapeutic strategies aimed to modify mRNA in DMD to generate a much milder disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DMD:
-
Duchenne muscular dystrophy
- BMD:
-
Becker muscular dystrophy
References
Emery AEH 1993 Duchenne muscular dystrophy. Oxford University Press, Oxford, U.K.
Bushby KM, Gardner-Medwin D 1993 The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. J Neurol 240: 98–104
Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, Province MA 1983 Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve 6: 91–103
Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM 1987 Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517
Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM 1988 An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2: 90–95
Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, Meng G, Muller CR, Lindlof M, Kaariainen H, de la Chapelle A, Kiuru A, Savontaus M-L, Gilgenkrantz H, Recan D, Chelly J, Kaplan J-C, Covone AE, Archidiacono N, Romeo G, Liechti-Gallati S, Schneider V, Braga S, Moser H, Darras BT, Murphy P, Francke U, Chen JD, Morgan G, Denton M, Greenberg CR, Wrogemann K, Blonden LAJ, van Paassen HMB, van Ommen GJB, Kunkel LM 1989 The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 45: 498–506
Chelly J, Gilgenkrantz H, Lambert M, Hamard G, Chafey P, Recan D, Katz P, de la Chapelle A, Koenig M, Ginjaar IB, Fardeau M, Tome F, Kahn A, Kaplan J-C 1990 Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell 63: 1239–1248
Shiga N, Takeshima Y, Sakamoto H, Inoue K, Yokota Y, Yokoyama M, Matsuo M 1997 Disruption of the splicing enhancer sequence within exon 27 of the dystrophin gene by a nonsense mutation induces partial skipping of the exon and is responsible for Becker muscular dystrophy. J Clin Invest 100: 2204–2210
Ginjaar IB, Kneppers AL, v d Meulen JD, Anderson LV, Bremmer-Bout M, van Deutekom JC, Weegenaar J, den Dunnen JT, Bakker E 2000 Dystrophin nonsense mutation induces different levels of exon 29 skipping and leads to variable phenotypes within one BMD family. Eur J Hum Genet 8: 793–796
Roberts RG, Barby TF, Manners E, Bobrow M, Bentley DR 1991 Direct detection of dystrophin gene rearrangements by analysis of dystrophin mRNA in peripheral blood lymphocytes. Am J Hum Genet 49: 298–310
Matsuo M, Masumura T, Nishio H, Nakajima T, Kitoh Y, Takumi T, Koga J, Nakamura H 1991 Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy Kobe. J Clin Invest 87: 2127–2131
Tuffery S, Chambert S, Bareil C, Sarda P, Coubes C, Echenne B, Demaille J, Claustres M 1998 Mutation analysis of the dystrophin gene in Southern French DMD or BMD families: from Southern blot to protein truncation test. Hum Genet 102: 334–342
Fajkusova L, Lukas Z, Tvrdikova M, Kuhrova VV, Hajek J, Fajkus J 2001 Novel dystrophin mutations revealed by analysis of dystrophin mRNA: alternative splicing suppresses the phenotypic effect of a nonsense mutation. Neuromuscul Disord 11: 133–138
Shapiro MB, Senapathy P 1987 RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15: 7155–7174
Nakai K, Sakamoto H 1994 Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene 141: 171–177
Ahn AH, Kunkel LM 1993 The structural and functional diversity of dystrophin. Nat Genet 3: 283–291
Nishio H, Takeshima Y, Narita N, Yanagawa H, Suzuki Y, Ishikawa Y, Minami R, Nakamura H, Matsuo M 1994 Identification of a novel first exon in the human dystrophin gene and of a new promoter located more than 500 kb upstream of the nearest known promoter. J Clin Invest 94: 1037–1042
Hagiwara Y, Nishio H, Kitoh Y, Takeshima Y, Narita N, Wada H, Yokoyama M, Nakamura H, Matsuo M 1994 A novel point mutation (G −1 to T) in a 5′ splice donor site of intron 13 of the dystrophin gene results in exon skipping and is responsible for Becker muscular dystrophy. Am J Hum Genet 54: 53–61
Ikezawa M, Minami N, Takahashi M, Goto Y, Miike T, Nonaka I 1998 Dystrophin gene analysis on 130 patients with Duchenne muscular dystrophy with a special reference to muscle mRNA analysis. Brain Dev 20: 165–168
Tuffery-Giraud S, Chambert S, Demaille J, Claustres M 1999 Point mutations in the dystrophin gene: evidence for frequent use of cryptic splice sites as a result of splicing defects. Hum Mutat 14: 359–368
Schwarze U, Starman BJ, Byers PH 1999 Redefinition of exon 7 in the COL1A1 gene of type I collagen by an intron 8 splice-donor-site mutation in a form of osteogenesis imperfecta: influence of intron splice order on outcome of splice-site mutation. Am J Hum Genet 65: 336–344
Attanasio C, de Moerloose P, Antonarakis SE, Morris MA, Neerman-Arbez M 2001 Activation of multiple cryptic donor splice sites by the common congenital afibrinogenemia mutation, FGA IVS4 + 1 G–>T. Blood 97: 1879–1881
Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, Ishihara T, Nonaka I, Ozawa E, Sugita H 1988 Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333: 861–863
Surono A, Takeshima Y, Wibawa T, Ikezawa M, Nonaka I, Matsuo M 1999 Circular dystrophin RNAs consisting of exons that were skipped by alternative splicing. Hum Mol Genet 8: 493–500
Angelini C, Beggs AH, Hoffman EP, Fanin M, Kunkel LM 1990 Enormous dystrophin in a patient with Becker muscular dystrophy. Neurology 40: 808–812
Toscano A, Vitiello L, Comi GP, Galvagni F, Miorin M, Prelle A, Fortunato F, Bardoni A, Mora M, Fiumara A, Falsaperla R, Tomelleri G, Tonin P, Danieli GA, Vita G 1995 Duplication of dystrophin gene and dissimilar clinical phenotype in the same family. Neuromuscul Disord 5: 475–481
Prior TW, Bartolo C, Papp AC, Snyder PJ, Sedra MS, Burghes AH, Mendell JR 1996 Nonsense mutations in a Becker muscular dystrophy and an intermediate patient. Hum Mutat 7: 72–75
Lenk U, Hanke R, Thiele H, Speer A 1993 Point mutations at the carboxy terminus of the human dystrophin gene: implications for an association with mental retardation in DMD patients. Hum Mol Genet 2: 1877–1881
Roberts RG, Gardner RJ, Bobrow M 1994 Searching for the 1 in 2,400,000: a review of dystrophin gene point mutations. Hum Mutat 4: 1–11
Sironi M, Pozzoli U, Cagliani R, Comi GP, Bardoni A, Bresolin N 2001 Analysis of splicing parameters in the dystrophin gene: relevance for physiological and pathogenetic splicing mechanisms. Hum Genet 109: 73–84
Hentze MW, Kulozik AE 1999 A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96: 307–310
Wibawa T, Takeshima Y, Mitsuyoshi I, Wada H, Surono A, Nakamura H, Matsuo M 2000 Complete skipping of exon 66 due to novel mutations of the dystrophin gene was identified in two Japanese families of Duchenne muscular dystrophy with severe mental retardation. Brain Dev 22: 107–112
Matsuo M 2002 Duchenne and Becker muscular dystrophy: from gene diagnosis to molecular therapy. IUBMB Life 53: 1–6
Melis MA, Muntoni F, Cau M, Loi D, Puddu A, Boccone L, Mateddu A, Cianchetti C, Cao A 1998 Novel nonsense mutation (C–>A nt 10512) in exon 72 of dystrophin gene leading to exon skipping in a patient with a mild dystrophinopathy. Hum Mutat ( suppl 1): S137–S138
Pramono ZA, Takeshima Y, Alimsardjono H, Ishii A, Takeda S, Matsuo M 1996 Induction of exon skipping of the dystrophin transcript in lymphoblast-oid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem Biophys Res Commun 226: 445–449
Takeshima Y, Yagi M, Ishikawa Y, Ishikawa Y, Minami R, Nakamura H, Matsuo M 2001 Oligonucleotides against a splicing enhancer sequence led to dystrophin production in muscle cells from a Duchenne muscular dystrophy patient. Brain Dev 23: 788–798
van Deutekom JCT, Bremmer-Bout M, Janson AAM, Ginjaar IB, Baas F, den Dunnen JT, van Ommen GJ 2001 Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet 10: 1547–1554
Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, Morgan JE, Partridge TA, Wilton SD 2001 Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci U S A 98: 42–47
Acknowledgements
The authors thank N. Kageyama for her help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant from the Ministry of Education, Science, Sports and Culture of Japan and a research grant (11B-1) from the Ministry of Health, Labor and Welfare, Japan.
Rights and permissions
About this article
Cite this article
Adachi, K., Takeshima, Y., Wada, H. et al. Heterogous Dystrophin mRNA Produced by a Novel Splice Acceptor Site Mutation in Intermediate Dystrophinopathy. Pediatr Res 53, 125–131 (2003). https://doi.org/10.1203/00006450-200301000-00021
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-200301000-00021
This article is cited by
-
Faulty RNA splicing: consequences and therapeutic opportunities in brain and muscle disorders
Human Genetics (2017)
-
A novel splicing silencer generated by DMD exon 45 deletion junction could explain upstream exon 44 skipping that modifies dystrophinopathy
Journal of Human Genetics (2014)
-
Mutation spectrum of the dystrophin gene in 442 Duchenne/Becker muscular dystrophy cases from one Japanese referral center
Journal of Human Genetics (2010)
-
Identification of seven novel cryptic exons embedded in the dystrophin gene and characterization of 14 cryptic dystrophin exons
Journal of Human Genetics (2007)
-
A nonsense mutation-created intraexonic splice site is active in the lymphocytes, but not in the skeletal muscle of a DMD patient
Human Genetics (2006)