Abstract
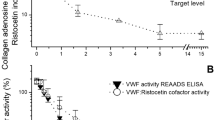
von Willebrand Factor (VWF) is important in platelet adhesion and shear-dependent platelet activation. We performed flow cytometric analyses of VWF binding to and activation of platelets from healthy neonates, children, and adults. Platelets from cord blood (n = 38; gestational age: 36–42 wk; birth weight: 2.4–5.1 kg), neonatal venous blood (n = 19; d 2–3 of life), children (n = 15; age: 1.5–16.3 y), and adults (n = 22; age: 18–55 y) were studied. Binding of VWF was assessed using an antihuman VWF polyclonal antibody and a FITC-conjugated secondary antibody. Platelet activation was determined by the expression of CD62P, CD63, CD41, CD42b, activated GPIIb/IIIa (PAC-1), procoagulant surface (as reflected by annexin V binding), and microparticle formation. Although the mean percentage of VWF-positive platelets was not significantly higher in unstimulated platelets from 2- to 3-d-old neonates, their platelets were more activated than those from adults, and there was a positive correlation of VWF binding with platelet activation (CD62P:r = 0.74, p < 0.001; annexin V:r = 0.46, p < 0.05). In adults, after in vitro activation of platelets with thrombin and ADP, VWF binding to platelets increased and correlated significantly with CD62P expression (r = 0.71, p < 0.001). VWF binding to unstimulated neonatal platelets was, however, higher than that to in vitro–stimulated platelets from adults at the same level of expression of platelet activation markers. Further studies are required to assess the mechanism and significance of VWF binding to activated platelets in the neonatal period.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CD:
-
cluster of differentiation
- CTAD:
-
0.38% sodium citrate anticoagulant supplemented with theophylline, adenosine, and dipyridamole
- MCF:
-
mean channel fluorescence
- MDCF:
-
median channel fluorescence
- MP:
-
platelet microparticles
- PE:
-
phycoerythrin
- PF:
-
paraformaldehyde
- VWF:
-
von Willebrand factor
References
Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L 1992 Maturation of the hemostatic system during childhood. Blood 80: 1998–2005
Andrew M 2000 Developmental hemostasis: relevance to thromboembolic complications in pediatric patients. In: Andrews M, Monagle PT, Brooker L (eds) Thromboembolic Complications During Infancy and Childhood. BC Decker, Hamilton, ON, Canada pp 5–46.
Hathaway W, Corrigan J 1991 Report of scientific and standardization subcommittee on neonatal hemostasis. Thromb Haemost 65: 323–325
Andrew M, Paes B, Milner R 1990 Development of the hemostatic system in the neonate and young infant. J Pediatr Hematol Oncol 12: 95–104
Andrew M, Paes B, Johnston M 1987 Development of the human coagulation system in the full term infant. Blood 70: 165–172
Andrew M, Mitchell L, Vegh P, Ofosu F 1994 Thrombin regulation in children differs from adults in the absence and presence of heparin. Thromb Haemost 72: 836–842
Schmidt B, Andrew M 1996 Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics 96: 939–943
Nowak-Göttl U, von Kries R, Göbel U 1997 Neonatal symptomatic thromboembolism in Germany: two year survey. Arch Dis Child 76: F163–F167
Andrew M 2000 Epidemiology of venous thromboembolic events. In: Andrews M, Monagle PT, Brooker L (eds) Thromboembolic Complications During Infancy and Childhood. BC Decker, Hamilton, ON, Canada, pp 111–164.
Andrew M 1997 The relevance of development hemostasis to hemorrhagic disorders of newborns. Semin Perinatol 21: 70–85
Ahmann PA, Lazzara A, Dykes FD, Brann AW, Schwartz JF 1980 Intraventricular hemorrhage in the high risk preterm infant: incidence and outcome. Ann Neurol 7: 118–124
DeSa DJ, MacLean BS 1970 An analysis of massive pulmonary haemorrhage in the newborn infant in Oxford, 1948–68. J Obstet Gynaecol Br Commonw 77: 158–163
Hayden CK, Shattuck KE, Richardson CJ, Ahrendt DK, House R, Swischuk LE 1985 Subependymal germinal matrix hemorrhage in full-term neonates. Pediatrics 75: 714–718
Rajasekhar D, Kestin AS, Bednarek FJ, Ellis PA, Barnard MR, Michelson AD 1994 Neonatal platelets are less reactive than adult platelets to physiological agonists in whole blood. Thromb Haemost 72: 953–963
Ts'ao CH, Green D, Schultz K 1976 Function and ultrastructure of platelets of neonates: enhanced ristocetin aggregation of neonatal platelets. Br J Haematol 32: 225–233
Mull MM, Hathaway WE 1970 Altered platelet function in newborns. Pediatr Res 4: 229–237
Andrew M, Paes B, Bowker J, Vegh P 1990 Evaluation of an automated bleeding time device in the newborn. Am J Hematol 335: 275–277
Sutor AH 1998 The bleeding time in pediatrics. Semin Thromb Hemost 24: 531–543
Carcao MD, Blanchette VS, Dean JA, He L, Kern MA, Stain AM, Sparling CR, Stephens D, Ryan G, Freedman J, Rand ML 1998 The Platelet Function Analyzer (PFA-100): a novel in-vitro system for evaluation of primary haemostasis in children. Br J Haematol 101: 70–73
Israels SJ, Cheang T, McMillan-Ward EM, Cheang M 2001 Evaluation of primary hemostasis in neonates with a new in vitro platelet function analyzer. J Pediatr 138: 116–119
Roschitz B, Sudi K, Koestenberger M, Muntean W 2001 Shorter PFA 100 closure times in neonates than in adults: role of red cells, white cells and von Willebrand factor. Acta Paediatr 90: 664–670
Sadler JE 1998 Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 67: 395–424
Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD 1986 Involvement of large plasma von Willebrand factor (VWF) multimers and unusually large VWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest 78: 1456–1461
McCrary JK, Nolasco LH, Hellums JD, Kroll MH, Turner NA, Moake JL 1995 Direct demonstration of radiolabeled von Willebrand factor binding to platelet glycoprotein Ib and IIb-IIIa in the presence of shear stress. Ann Biomed Eng 23: 787–793
Ruggeri ZM 1999 Structure and function of von Willebrand factor. Thromb Haemost 82: 576–584
Keularts IM, Hamulyak K, Hemker HC, Beguin S 2000 The effect of DDAVP infusion on thrombin generation in platelet-rich plasma of von Willebrand Type 1 and mild haemophilia A patients. Thromb Haemost 84: 638–642
Katz JA, Moake JL, McPherson PD, Weinstein MJ, Moise KJ, Carpenter RJ, Sala DJ 1989 Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood 73: 1851–1858
Weinstein J, Blanchard R, Moake J, Vosburgh E, Moise K 1989 Fetal and neoantal von Willebrand factor is unusually large and similar to the VWF in patients with TTP. Br J Haematol 72: 68–72
Shenkman B, Linder N, Savion N, Tamarin I, Dardik R, Kennet G, German B, Varon D 1999 Increased neonatal platelet deposition on subendothelium under flow conditions: the role of plasma von Willebrand factor. Pediatr Res 45: 270–275
Kühne T, Hornstein A, Chang W, Semple J, Blanchette V, Freedman J 1995 Flow cytometric evaluation of platelet activation in blood collected into EDTA vs. Diatube H, a sodium citrate solution supplemented with theophylline, adenosine, and dipyridamole. Am J Hematol 50: 40–45
Ledford MR, Kent JW, Civantos F 1990 A comparative study of three methods for the visualization of von Willebrand factor multimers. Thromb Haemost 64: 569–575
Aihara M, Sawada Y, Ueno K, Morimoto S, Yoshida Y, de Serres M, Cooper HA, Wagner RH 1986 Visualization of von Willebrand factor multimers by immunoenzymatic stain using avidin-biotin peroxidase complex. Thromb Haemost 55: 263–267
Freedman J, Mody M, Lazarus AH, Dewar L, Song S, Blanchette VS, Garvey MB, Ofosu FA 2002 Platelet activation and hypercoagulability following treatment with porcine factor VIII (HYATE:C). Am J Hematol 69: 192–199
Abrams CS, Ellison N, Budzynski AZ, Shattil SJ 1990 Direct detection of activated platelets and platelet-derived microparticles in humans. Blood 75: 128–138
Chow TW, Turner NA, Chintagumpala M, McPherson PD, Nolasco LH, Rice L, Hellums JD, Moake JL 1998 Increased von Willebrand factor binding to platelets in single episode and recurrent types of thrombotic thrombocytopenic purpura. Am J Hematol 57: 293–302
Andrews RK, Booth WJ, Gorman JJ, Castaldi PA, Berndt MC 1989 Purification of botrocetin from Bothrops jararaca venom. Analysis of the botrocetin-mediated interaction between von Willebrand factor and the human platelet membrane glycoprotein Ib-IX complex. Biochemistry 28: 8317–8326
Girma JP, Takahashi Y, Yoshioka A, Diaz J, Meyer D 1990 Ristocetin and botrocetin involve two distinct domains of von Willebrand factor for binding to platelet membrane glycoprotein Ib. Thromb Haemost 64: 326–332
Chang H, Mody M, Lazarus AH, Ofosu F, Garvey MB, Blanchette V, Teitel J, Freedman J 1998 Platelet activation induced by porcine factor VIII (Hyate:C). Am J Hematol 57: 200–205
Song S, Mody M, Freedman J, Ellis J, Lazarus AH 2003 von Willebrand factor (VWF)-dependent human platelet activation: porcine VWF utilizes different transmembrane signaling pathways than does thrombin to activate platelets, but both require protein phosphatase function. J Thromb Haemost 1: 337–346
Parker RI, Gralnick HR 1986 Identification of platelet glycoprotein IIb/IIIa as the major binding site for released platelet-von Willebrand factor. Blood 68: 732–736
Gralnick HR, Williams SB, McKeown LP, Magruder L, Hansmann K, Vail M, Parker RI 1991 Platelet von Willebrand factor. Mayo Clin Proc 66: 634–640
Wagner DD 1993 The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost 70: 105–110
Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E 2001 Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood 98: 2730–2735
Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Ginsburg D, Tsai HM 2001 Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413: 488–494
Sugimoto M, Mohri H, McClintock RA, Ruggeri ZM 1991 Identification of discontinuous von Willebrand factor sequences involved in complex formation with botrocetin. A model for the regulation of von Willebrand factor binding to platelet glycoprotein Ib. J Biol Chem 266: 18172–18178
Kulkarni S, Dopheide SM, Yap CL, Ravanat C, Freund M, Mangin P, Heel KA, Street A, Harper IS, Lanza F, Jackson SP 2000 A revised model of platelet aggregation. J Clin Invest 105: 783–791
Kasirer-Friede A, Ware J, Leng L, Marchese P, Ruggeri ZM, Shattil SJ 2002 Lateral clustering of platelet GP Ib-IX complexes leads to up-regulation of the adhesive function of integrin alpha IIb beta 3. J Biol Chem 277: 11949–11956
Hurtaud-Roux MF, Hezard N, Lefranc V, Foucart C, Desanges C, Aujard Y, Simon G, Cand M, Rambaldini A, Droulle C, N'Guyen P, Potron G, Schlegel N 2001 Quantification of the major integrins and p-selectin in neonatal platelets by flow cytometry. A bi-centric study. Thromb Haemost 86: 284a
Simak J, Holada K, Janota J, Stranak Z 1999 Surface expression of major membrane glycoproteins on resting and TRAP-activated neonatal platelets. Pediatr Res 46: 445–449
Ruggeri ZM, De Marco L, Gatti L, Bader R, Montgomery RR 1983 Platelets have more than one binding site for von Willebrand factor. J Clin Invest 72: 1–12
Pietrucha T, Wojciechowski T, Greger J, Jedrzejewska E, Nowak S, Chrul S, Golanski J, Watala C 2001 Differentiated reactivity of whole blood neonatal platelets to various agonists. Platelets 12: 99–107
Gatti L, Guarneri D, Caccamo ML, Gianotti GA, Marini A 1996 Platelet activation in newborns detected by flow-cytometry. Biol Neonate 70: 322–327
Tanindi S, Kurekci AE, Koseoglu V, Kurt M, Ozcan O 1995 The normalization period of platelet aggregation in newborns. Thromb Res 80: 57–62
Acknowledgements
The authors thank Dr. Lillian Sung for help with statistical analysis and Dr. Sara Israels for helpful discussions during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented, in part, at the 15th Annual Meeting of the American Society of Pediatric Hematology/Oncology, May 2002, Baltimore, MD, U.S.A.
Rights and permissions
About this article
Cite this article
Schmugge, M., Rand, M., Bang, K. et al. The Relationship of von Willebrand Factor Binding to Activated Platelets from Healthy Neonates and Adults. Pediatr Res 54, 474–479 (2003). https://doi.org/10.1203/01.PDR.0000081294.26060.4B
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000081294.26060.4B
This article is cited by
-
The role of the calibrated automated thrombogram in neonates: describing mechanisms of neonatal haemostasis and evaluating haemostatic drugs
European Journal of Pediatrics (2022)
-
A review of the role of extracellular vesicles in neonatal physiology and pathology
Pediatric Research (2021)
-
Thrombocytosis in preterm infants: a possible involvement of thrombopoietin receptor gene expression
Journal of Molecular Medicine (2005)