Abstract
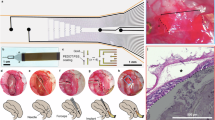
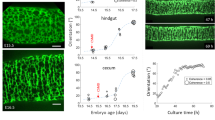
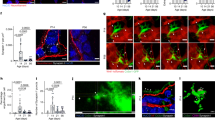
Intestinal motility disorders are a major cause of morbidity after surgical repair of intestinal atresia of unknown mechanism. We hypothesized that interruption of antenatal peristalsis may disturb the normal development of the enteric nervous system. Using a series of neuronal (synaptophysin, neuronal nitric oxide synthase, neurofilaments) and nonneuronal markers (glial acidic fibrillary protein and c-Kit) and immunohistochemistry, we have defined developmental steps of the enteric nervous system in normal intestine (12 fetuses, 15 children, and 4 adults) and their alterations above and below the obstacle in 22 human intestinal atresia compared with age-matched controls. Antisynaptophysin antibody revealed the progressive conversion of the myenteric plexus from a continuous belt into regularly spaced ganglions during normal fetal gut development and, by contrast, the significantly delayed appearance of individual neuronal ganglions in the distal segments of atresia (p < 0.05). Staging using three other markers for neuronal (neurofilaments and neuronal nitric oxide synthase) and nonneuronal cells (glial acidic fibrillary protein) confirmed that maturation of the myenteric plexus was significantly delayed below atresia (p < 0.01). These results indicate that intestinal atresia impairs the development of the enteric nervous system and provide an anatomical substrate for the motility disorders observed after surgical repair. They point to the role of peristalsis in normal gut development and suggest that stimulation of peristalsis might be used to accelerate recovery.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ENS:
-
enteric nervous system
- GFAP:
-
glial fibrillary acidic protein
- ICC:
-
interstitial cells of Cajal
- NF:
-
neurofilament
- nNOS:
-
neuronal nitric oxide synthase
- SIS:
-
staining index for synaptophysin
References
Cragan JD, Martin ML, Moore CA, Khoury MJ 1993 Descriptive epidemiology of small intestinal atresia, Atlanta, Georgia. Teratology 48: 441–450
Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Engum SA 1998 Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg 133: 490–496
Masumoto K, Suita S, Nada O, Taguchi T, Guo R, Yamanouchi T 1999 Alterations of the intramural nervous distributions in a chick intestinal atresia model. Pediatr Res 45: 30–37
Watanabe Y, Ando H, Seo T, Katsuno S, Marui Y, Horisawa M 2001 Two-dimensional alterations of myenteric plexus in jejunoileal atresia. J Pediatr Surg 36: 474–478
Goyal RK, Hirano I 1996 The enteric nervous system. N Engl J Med 334: 1106–1115
Gershon MD, Chalazonitis A, Rothman TP 1993 From neural crest to bowel: development of the enteric nervous system. J Neurobiol 24: 199–214
Grand RJ, Watkins JB, Torti FM 1976 Development of the human gastrointestinal tract. A review. Gastroenterology 70: 790–810
Trahair JF 1993 Is fetal enteral nutrition important for normal gastrointestinal growth?: a discussion. JPEN J Parenter Enteral Nutr 17: 82–85
Surana R, Puri P 1994 Small intestinal atresia: effect on fetal nutrition. J Pediatr Surg 29: 1250–1252
Tepas JJ, Wyllie RG, Shermeta DW, Inon AE, Pickard LR, Haller JA Jr 1979 Comparison of histochemical studies of intestinal atresia in the human newborn and fetal lamb. J Pediatr Surg 14: 376–380
Doolin EJ, Ormsbee HS, Hill JL 1987 Motility abnormality in intestinal atresia. J Pediatr Surg 22: 320–324
Grunnet ML 1995 A lectin and synaptophysin study of developing brain. Pediatr Neurol 13: 157–160
Hitchcock RJ, Pemble MJ, Bishop AE, Spitz L, Polak JM 1992 Quantitative study of the development and maturation of human oesophageal innervation. J Anat 180: 175–183
Bredt DS, Hwang PM, Snyder SH 1990 Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770
Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG 1990 Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347
Vanderwinden JM, De Laet MH, Schiffmann SN, Mailleux P, Lowenstein CJ, Snyder SH, Vanderhaeghen JJ 1993 Nitric oxide synthase distribution in the enteric nervous system of Hirschsprung's disease. Gastroenterology 105: 969–973
Timmermans JP, Barbiers M, Scheuermann DW, Bogers JJ, Adriaensen D, Fekete E, Mayer B, Van Marck EA, De Groodt-Lasseel MH 1994 Nitric oxide synthase immunoreactivity in the enteric nervous system of the developing human digestive tract. Cell Tissue Res 275: 235–245
Eaker EY, Sallustio JE, Harris JM, Shaw G 1993 Myenteric plexus neurons have developmentally acquired differences in the medium molecular weight subunit of neurofilament protein. Neuroscience 53: 561–570
Brandt CT, Graham A, Tam PK 1997 Densities of nitric oxide synthesizing nerves in smooth muscles of human gut during fetal development. J Pediatr Surg 32: 1314–1317
Wester T, O'Briain DS, Puri P 1999 Notable postnatal alterations in the myenteric plexus of normal human bowel. Gut 44: 666–674
Jessen KR, Mirsky R 1980 Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 286: 736–737
Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A 1995 W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349
Burns AJ, Douarin NM 1998 The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125: 4335–4347
Ullian EM, Sapperstein SK, Christopherson KS, Barres BA 2001 Control of synapse number by glia. Science 291: 657–661
Wester T, Eriksson L, Olsson Y, Olsen L 1999 Interstitial cells of Cajal in the human fetal small bowel as shown by c-kit immunohistochemistry. Gut 44: 65–71
Kenny SE, Connell G, Woodward MN, Lloyd DA, Gosden CM, Edgar DH, Vaillant C 1999 Ontogeny of interstitial cells of Cajal in the human intestine. J Pediatr Surg 34: 1241–1247
Bisset WM, Watt JB, Rivers RP, Milla PJ 1988 Ontogeny of fasting small intestinal motor activity in the human infant. Gut 29: 483–488
Ekblad E, Sjuve R, Arner A, Sundler F 1998 Enteric neuronal plasticity and a reduced number of interstitial cells of Cajal in hypertrophic rat ileum. Gut 42: 836–844
Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G 1999 Plasticity in the enteric nervous system. Gastroenterology 117: 1438–1458
Farge E 2003 Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol 13: 1365–1377
Del Riccio V, van Tuyl M, Post M 2003 Apoptosis in lung development and neonatal lung injury. Pediatr Res 55: 183–189
Berseth CL 1989 Gestational evolution of small intestine motility in preterm and term infants. J Pediatr 115: 646–651
Acknowledgements
The authors thank G. Pivert for technical assistance and T. Attié for helpful discussion.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Supported by the Fondation de l'Avenir, the Association pour l'Etude de la Pathologie Pédiatrique, and the Fondation NRJ-Institut de France.
The Group for the Study of Intestinal Atresia from the Departments of Pediatric Surgery and Pathology of Hôpital Edouard Herriot, Lyon (J.P. Chappuis, R. Bouvier); Hôpital d'Enfants-La Timone, Marseille (J.M. Guys, J.F. Pellissier); Hôpital Gatien de Clocheville, Tours (H. Lardy, F. Fetissof); Hôpital Pontchaillou, Rennes (O. Azzis, M.P. Ramée); Hôpital Mère-Enfants, Nantes (G. Podevin, C. Laboisse); Hôpital Robert Debré, Paris (Y. Aigrain, M. Peuchmaur).
Rights and permissions
About this article
Cite this article
Khen, N., Jaubert, F., Sauvat, F. et al. Fetal Intestinal Obstruction Induces Alteration of Enteric Nervous System Development in Human Intestinal Atresia. Pediatr Res 56, 975–980 (2004). https://doi.org/10.1203/01.PDR.0000145294.11800.71
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000145294.11800.71
This article is cited by
-
Analysis of enteric nervous system and intestinal epithelial barrier to predict complications in Hirschsprung’s disease
Scientific Reports (2020)
-
Physiopathology of vesico-ureteral reflux
Italian Journal of Pediatrics (2016)
-
Erythromycin establishes early oral feeding in neonates operated for congenital intestinal atresias
Pediatric Surgery International (2009)
-
Host Factors in Amniotic Fluid and Breast Milk that Contribute to Gut Maturation
Clinical Reviews in Allergy & Immunology (2008)