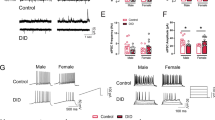
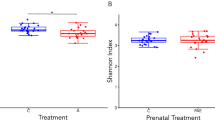
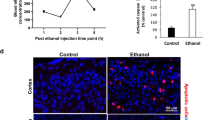
Abstract
Alcohol is one of the most common noxious substance to which fetuses are exposed. The aim of the study was to determine the effects of in utero alcohol exposure on excitotoxin-induced neuronal migration disorders. Female hamsters received alcohol (7%) for 3–5 mo or for the last 9–12 d of gestation. Alcohol diet was continued for 5 d during lactation in both groups. Drinking behavior was monitored. Peak plasma alcohol levels were 104 ± 12 mg/dL and 225 ± 6 mg/dL after 30 min for hamsters receiving an intragastric dose of 3 mL or 5 mL alcohol, respectively. At birth, pups received intrapallial injections ibotenic acid (1 ng, 100 ng, or 10 μg). Histology and N-methyl-d-aspartic acid (NMDA) receptor labeling by 3H-MK-801 in the pups cortices were studied. Short-term-alcohol-exposed pups had normal body and brain weights at birth, but their body growth was retarded postnatally. Ibotenic acid induced similar neuronal migration impairments in control and alcohol-exposed pups (nodular heterotopia in the white matter and/or deep cortical layers, subpial ectopia, and micro- or polymicrogyria). The size of lesions induced by 100 ng ibotenic acid was increased in alcohol-exposed pups; the 10 μg dose was lethal. The density of 3H-MK-801 binding sites was similar in the three groups, indicating that exacerbated ibotenic acid excitotoxicity in alcohol-exposed pups did not result from increased NMDA receptor density. This study shows that alcohol exposure at levels that do not induce neuron migration disorders is sufficient to enhance the effects of the hypoxia-ischemia mimicking effects of ibotenic acid.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ARND:
-
alcohol-related neurodevelopmental disorders
- FAS:
-
fetal alcohol syndrome
- GABA:
-
gamma-aminobutyric acid
- GFAP:
-
glial fibrillary acidic protein
- 3H-MK-801:
-
tritiated dizocilpine
- NeuN:
-
neuronal nuclear antigen
- NGF:
-
nerve growth factor
- NR2B:
-
NMDA receptor subunit encoded by the GRIN-2B gene
- NMDA:
-
N-methyl-d-aspartic acid
- PAF:
-
platelet-activating factor
References
Lemoine P, Harrousseau H, Borteyru J, Menuet J 1968 Les enfants de parents alcooliques: anomalies observées: à propos de 127 cas. Ouest Médical 25: 76–482
Jones KL, Smith AW 1973 Recognition of the fetal alcohol syndrome in early infancy. Lancet 2: 99–1001
Stratton K, Howe C, Battaglia F (eds) 1996 Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. National Academy Press, Washington, DC, pp 4–21
Clarren SK, Alvord EC Jr, Sumi SM, Streissguth AP, Smith DW 1978 Brain malformations related to pre-natal exposure to ethanol. J Pediatr 92: 4–67
Wisniewski K, Dambska M, Sher JH, Qazi Q 1983 A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatrics 14: 97–201
Clark CM, Li D, Conry J, Conry R, Loock C 2000 Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics 105: 096–1099
Larroque B, Kaminski M, Dehaene P, Subtil D, Delfosse MJ, Querleu D 1995 Moderate prenatal alcohol exposure and psychomotor development at preschool age. Am J Public Health 85: 654–1661
Sampson PD, Streissguth AP, Bookstein FL, Barr HM 2000 On categorizations in analyses of alcohol teratogenesis. Environ Health Perspect 108: 21–428
Miller MW 1993 Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res 17: 04–314
Gressens P, Lammens M, Picard JJ, Evrard P 1992 Ethanol-induced disturbances of gliogenesis and neurogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcoholism 27: 19–226
Lovinger D, White G, Weight FF 1989 Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 721–1724
Aguayo LG 1990 Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur J Pharmacol 187: 27–130
Komuro H, Rakic P 1993 Modulation of neuronal migration by NMDA receptors. Science 260: 5–97
Gressens P, Baes M, Leroux P, Lombet A, Van Veldhoven P, Janssen A, Vamecq J, Marret S, Evrard P 2000 Neuronal migration disorder in Zellweger mice is secondary to glutamate receptor dysfunction. Ann Neurol 48: 36–343
Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW 2000 Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287: 056–1060
Guerri C, Pascual M, Renau-Piqueras J 2001 Glia and fetal alcohol syndrome. Neurotoxicology 22: 93–599
Miller MW, Robertson S 1993 Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J Comp Neurol 337: 53–266
Guerri C, Renau-Piqueras J 1997 Alcohol, astroglia and brain development. Mol Neurobiol 15: 5–81
Marret S, Gressens P, Evrard P 1996 Arrest of neuronal migration by excitatory amino acids in hamster developing brain. Proc Natl Acad Sci U S A 93: 5463–15468
Barkovich AJ, Gressens P, Evrard P 1992 Formation, maturation and disorders of brain neocortex. Am J Neuroradiol 13: 23–446
Kalluri HS, Mehta AK, Ticku MK 1998 Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res 58: 21–224
Naassila M, Daoust M 2002 Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem 80: 50–860
Chandler LJ, Sutton G, Norwood D, Sumners C, Crews FT 1997 Chronic ethanol increases N-methyl-d-aspartate-stimulated nitric oxide formation but not receptor density in cultured cortical neurons. Mol Pharmacol 51: 33–740
Liesi P 1997 Ethanol-exposed central neurons fail to migrate and undergo apoptosis. J Neurosci Res 48: 39–448
Charness ME, Safran RM, Perides G 1994 Ethanol inhibits neural cell-cell adhesion. J Biol Chem 269: 304–9309
Hirai K, Yoshioka H, Kihara M, Hasegawa K, Sawada T, Fushiki S 1999 Effects of ethanol on neuronal migration and neural cell adhesion molecules in the embryonic rat cerebral cortex: a tissue culture study. Brain Res Dev Brain Res 118: 05–210
Miller MW, Luo J 2002 Effects of ethanol and transforming growth factor beta (TGF beta) on neuronal proliferation and nCAM expression. Alcohol Clin Exp Res 26: 281–1285
Miller MW 1997 Effects of prenatal exposure to ethanol on callosal projection neurons in rat somatosensory cortex. Brain Res 766: 21–128
Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A 1998 Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet 19: 33–339
Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H 1997 Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science 278: 98–701
Hennebert O, Marret S, Carmeliet P, Gressens P, Laquerrière A, Leroux P 2004 Role of tissue derived plasminogen activator (t-PA) in an excitotoxic mouse model of neonatal white matter lesions. J Neuropathol Exp Neurol 63: 3–63
Gressens P, Muaku SM, Besse L, Nsegbe E, Gallego J, Delpech B, Gaultier C, Evrard P, Ketelslegers JM, Maiter D 1997 Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res Dev Brain Res 103: 1–35
Murillo-Fuentes ML, Murillo ML, Carreras O 2003 Effects of maternal ethanol consumption during pregnancy or lactation on intestinal absorption of folic acid in suckling rats. Life Sci 73: 199–2209
Ho PI, Ortiz D, Rogers E, Shea TB 2002 Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res 70: 94–702
Abel EL, Hannigan JH 1995 Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol 17: 445–462 (erratum p 689)
Lipton SA, Rosenberg PA 1994 Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330: 13–622
Acknowledgements
The authors thank Prof. Jean Costentin for allowing us to use the radio-imager and the drinking monitoring device, Dr. Jean François Ménard for his help in statistical analyses, M. Jean Paul Henry for his skillful handling of the animals, and Ms. Cathy Vendeville for her expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Institut de Recherches scientifiques sur les Boissons (IREB), Paris, France, and the Région Haute-Normandie.
Rights and permissions
About this article
Cite this article
Adde-Michel, C., Hennebert, O., Laudenbach, V. et al. Effect of Perinatal Alcohol Exposure on Ibotenic Acid-Induced Excitotoxic Cortical Lesions in Newborn Hamsters. Pediatr Res 57, 287–293 (2005). https://doi.org/10.1203/01.PDR.0000148712.30716.9D
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000148712.30716.9D
This article is cited by
-
Losing your nerves? Maybe it's the antibodies
Nature Reviews Immunology (2009)