Abstract
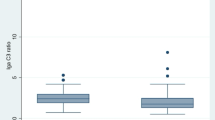
The charge selectivity (CS) function in human renal disease has not been unequivocally demonstrated to date. However, the clearance ratio of IgA to IgG may be theoretically useful in estimating CS in humans, since IgA and IgG have similar sizes and tertiary structures, but distinct isoelectric points (3.5–5.5 [IgA] and 4.5–9.0 [IgG]), and Stokes-Einstein radius: 61 Å (IgA) and 49–60 Å (IgG). Two-dimensional electrophoresis with the following immunoblotting revealed that the considerably anionic portion (isoelectric points [pI] <4.0) of IgA, visible in serum, was absent in the urine in steroid-sensitive nephrotic syndrome (SSNS) but present in the same during IgA nephropathy (IgAN) and membranoproliferative glomerulonephritis (MPGN). A latex assay revealed the CS index (CSI) was significantly low in patients with podocyte disease (group A), including SSNS, focal and segmental glomerulosclerosis (FSGS) and Finnish-type congenital nephrotic syndrome (FCNS), but high in those with Alport syndrome (AS), IgAN, Henoch-Schönlein purpura nephritis (HSPN), and MPGN (group B). The linear regression analysis of the IgA size selectivity index (IgA SSI; clearance ratio of IgA to transferrin) and SSI (clearance ratio of IgG to transferrin), which represents the clearance ratio of IgA to IgG referring to the transferrin clearance, revealed the influence of the charge more accurately. Indeed, the slope of the regression lines of IgA SSI (y) to SSI (x) were concluded to be y = 0.39x (group A) and y = 1.05x (group B), respectively. These results suggested that the charge selective barrier among podocyte diseases (group A) is preserved to some degree, but lost in cases of nephritis and AS (group B).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AS:
-
Alport syndrome
- CSI:
-
charge selectivity index
- FCNS:
-
Finnish-type congenital nephrotic syndrome
- FSGS:
-
focal and segmental glomerulosclerosis
- GBM:
-
glomerular basement membrane
- GCW:
-
glomerular capillary wall
- IgAN:
-
IgA nephropathy
- IgA SSI:
-
IgA size selectivity index
- MPGN:
-
membranoproliferative glomerulonephritis
- SSNS:
-
steroid-sensitive
References
Kanwar YS 1984 Biophysiology of glomerular filtration and proteinuria. Lab Invest 51: 7–21
Tryggvason K 1999 Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10: 2440–2445
Deen WM, Lazzara MJ, Myers BD 2001 Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–596
Brenner BM, Hostetter TH, Humes HD 1978 Molecular basis of proteinuria of glomerular origin. N Engl J Med 298: 826–833
Guasch A, Deen WM, Myers BD 1993 Charge selectivity of the glomerular filtration barrier in healthy and nephrotic humans. J Clin Invest 92: 2274–2282
Chang RL, Deen WM, Robertson CR, Brenner BM 1975 Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int 8: 212–218
Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, Brenner BM 1978 Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest 61: 72–78
Rennke HG, Patel Y, Venkatachalam MA 1978 Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int 13: 278–288
Olson JL, Rennke HG, Venkatachalam MA 1981 Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab Invest 44: 271–279
Hemmelder MH, de Zeeuw D, de Jong PE 1997 Measurement of glomerular charge selectivity in non-diabetic renal disease. Nephrol Dial Transplant 12: 57–62
Osicka TM, Comper WD 1998 Tubular inhibition destroys charge selectivity for anionic and neutral horseradish peroxidase. Biochim Biophys Acta 1381: 170–178
Birn H, Vorum H, Verroust PJ, Moestrup SK, Christensen EI 2000 Receptor-associated protein is important for normal processing of megalin in kidney proximal tubules. J Am Soc Nephrol 11: 191–202
Bolton GR, Deen WM, Daniels BS 1998 Assessment of the charge selectivity of glomerular basement membrane using Ficoll sulfate. Am J Physiol 274: F889–F896
Sorensson J, Ohlson M, Lindstrom K, Haraldsson B 1998 Glomerular charge selectivity for horseradish peroxidase and albumin at low and normal ionic strengths. Acta Physiol Scand 163: 83–91
Ohlson M, Sorensson J, Haraldsson B 2001 A gel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol 280: F396–F405
Smithies O 2003 Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci U S A 100: 4108–4113
Edwards A, Daniels BS, Deen WM 1999 Ultrastructural model for size selectivity in glomerular filtration. Am J Physiol 276: F892–F902
Greive KA, Nikolic-Paterson DJ, Guimaraes MA, Nikolovski J, Pratt LM, Mu W, Atkins RC, Comper WD 2001 Glomerular permselectivity factors are not responsible for the increase in fractional clearance of albumin in rat glomerulonephritis. Am J Pathol 159: 1159–1170
Guimaraes MA, Nikolovski J, Pratt LM, Greive K, Comper WD 2003 Anomalous fractional clearance of negatively charged Ficoll relative to uncharged Ficoll. Am J Physiol Renal Physiol 285: F1118–F1124
Takahashi S, Wada N, Harada K, Nagata M 2004 Cationic charge-preferential IgG reabsorption in the renal proximal tubules. Kidney Int 66: 1556–1560
Rudikoff S, Potter M, Segal DM, Padlan EA, Davies DR 1972 Crystals of phosphorylcholine-binding Fab-fragments from mouse myeloma proteins: preparation and x-ray analysis. Proc Natl Acad Sci U S A 69: 3689–3692
Kakuhara T, Hanzawa S, Nakayama S, Kanaguchi T, Ohara K 1975 Reassessment of selectivity index in nephrotic syndrome with special reference to the clinical significance of a small-molecular IgG fraction in the urine. Contrib Nephrol 4: 83–95
Ellis D, Buffone GJ 1981 Protein clearances and selectivity determinations in childhood nephrosis: a reappraisal. Clin Chem 27: 1397–1400
Sako M, Nakanishi K, Obana M, Yata N, Hoshii S, Takahashi S, Wada N, Takahashi Y, Kaku Y, Satomura K, Ikeda M, Honda M, Iijima K, Yoshikawa N 2005 Analysis of NPHS1, NPHS2, ACTN4, and WT1 in Japanese patients with congenital nephrotic syndrome. Kidney Int 67: 1248–1255
Gorg A, Postel W, Gunther S, Friedrich C 1988 Horizontal two-dimensional electrophoresis with immobilized pH gradients using PhastSystem. Electrophoresis 9: 57–59
Wada N, Ueda Y, Iidaka K, Inage Z, Kikkawa Y, Kitagawa T 1990 Portions of basement membrane with decreased negative charge in various glomerulonephritis. Clin Nephrol 34: 9–16
Sakagami Y, Nakajima M, Takagawa K, Ueda T, Akazawa H, Maruhashi Y, Shimoyama H, Kamitsuji H, Yoshioka A 2004 Analysis of glomerular anionic charge status in children with IgA nephropathy using confocal laser scanning microscopy. Nephron Clin Pract 96: c96–c104
Lee HS, Choi Y, Lee JS, Yu BH, Koh HI 1989 Ultrastructural changes in IgA nephropathy in relation to histologic and clinical data. Kidney Int 35: 880–886
Nomura Y, Ohya N, Shimada M 1989 A histopathological study on the prognosis of childhood IgA nephropathy and glomerular basement membrane lesions. Pediatr Nephrol 3: 242–247
Hattori S, Karashima S, Furuse A, Terashima T, Hiramatsu M, Murakami M, Matsuda I 1985 Clinicopathological correlation of IgA nephropathy in children. Am J Nephrol 5: 182–189
Hiraoka M, Takeda N, Tsukahara H, Kimura K, Takagi K, Hayashi S, Kato E, Ohta K, Sudo M 1995 Favorable course of steroid-responsive nephrotic children with mild initial attack. Kidney Int 47: 1392–1393
Ramjee G, Coovadia HM, Adhikari M 1997 Comparison of noninvasive methods for distinguishing steroid-sensitive nephrotic syndrome from focal glomerulosclerosis. J Lab Clin Med 129: 47–52
Caulfield JP, Farquhar MG 1976 Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A 73: 1646–1650
Kanwar YS, Linker A, Farquhar MG 1980 Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol 86: 688–693
Fujigaki Y, Nagase M, Hidaka S, Matsui K, Shirai M, Nosaka H, Kawachi H, Shimizu F, Hishida A 1998 Altered anionic GBM components in monoclonal antibody against slit diaphragm-injected proteinuric rats. Kidney Int 54: 1491–1500
Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B 2003 Glomerularfiltration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol Renal Physiol 284: F1226–F1234
Lahdenkari AT, Kestila M, Holmberg C, Koskimies O, Jalanko H 2004 Nephrin gene (NPHS1) in patients with minimal change nephrotic syndrome (MCNS). Kidney Int 65: 1856–1863
Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM 2003 Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C 2000 NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354
Tryggvason K, Wartiovaara J 2001 Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549
Jeansson M, Haraldsson B 2003 Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol 14: 1756–1765
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a Grant-in-Aid from the Japanese Society for Promotion of Science (17591118 to S. Takahashi).
Rights and permissions
About this article
Cite this article
Takahashi, S., Watanabe, S., Wada, N. et al. Charge Selective Function in Childhood Glomerular Diseases. Pediatr Res 59, 336–340 (2006). https://doi.org/10.1203/01.pdr.0000196733.47083.81
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000196733.47083.81
This article is cited by
-
Low molecular weight fucoidan ameliorates the inflammation and glomerular filtration function of diabetic nephropathy
Journal of Applied Phycology (2017)
-
Reevaluation of glomerular charge selective protein-sieving function
Pediatric Nephrology (2009)