Abstract
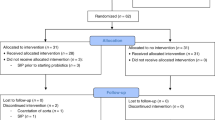
Antibiotics exert deleterious effects on the intestinal microbiota, favoring the emergence of opportunistic bacteria and diarrhea. Prebiotics are nondigestible food components that stimulate the growth of bifidobacteria. Our aim was to evaluate the effects on the intestinal microbiota of a prebiotic-supplemented milk formula after an antibiotic treatment. A randomized, double-blind, controlled clinical trial was carried out in 140 infants 1–2 y of age distributed into two groups after a 1-wk amoxicillin treatment (50 mg/kg/d) for acute bronchitis. The children received for 3 wk >500 mL/d of a formula with prebiotics (4.5 g/L) or a control without prebiotics. Fecal samples were obtained on d –7 (at the beginning of the antibiotic treatment), on d 0 (end of the treatment and before formula administration), and on d 7 and 21 (during formula administration). Counts of Bifidobacterium, Lactobacillus-Enterococcus, Clostridium lituseburiense cluster, Clostridium histolyticum cluster, Escherichia coli, and Bacteroides-Prevotella were evaluated by fluorescent in situ hybridization (FISH) and flow cytometry. Tolerance and gastrointestinal symptoms were recorded daily. Amoxicillin decreased total fecal bacteria and increased E. coli. The prebiotic significantly increased bifidobacteria from 8.17 ± 1.46 on d 0 to 8.54 ± 1.20 on d 7 compared with the control 8.22 ± 1.24 on d 0 versus 7.95 ± 1.54 on d 7. The Lactobacillus population showed a similar tendency while the other bacteria were unaffected. No gastrointestinal symptoms were detected during the prebiotic administration. Prebiotics in a milk formula increase fecal bifidobacteria early after amoxicillin treatment without inducing gastrointestinal symptoms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- FISH:
-
fluorescent in situ hybridization
- FOS:
-
fructooligosaccharides
References
Fanaro S, Chierici R, Guerrini P, Vigi V 2003 Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 441: 48–55
Tannock GW 1999 A fresh look at the intestinal microflora. In: Tannock GW (ed) Probiotics: a critical review. Horizon Scientific Press, Norfolk, pp 5–14
Garrido D, Suau A, Pochart P, Cruchet S, Gotteland M 2005 Modulation of the fecal microbiota by the intake of a Lactobacillus johnsonii La1-containing product in human volunteers. FEMS Microbiol Lett 248: 249–256
Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Doré J 1999 Direct analysis of genes encoding 16SrRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol 65: 4799–4807
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW 2000 Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30: 61–67
Brunser O, Figueroa G, Gotteland M, Haschke-Becher E, Magliola C, Cruchet S, Gibson GR, Palframan R, Rochat F, Chauffard F, Haschke F 2005 Effect of probiotic or prebiotic supplemented milk formulae on fecal microflora in infants. Asia Pac J Clin Nutr in press
Giuliano M, Barza M, Jacobus NV, Gorbach SL 1987 Effect of broad-spectrum parenteral antibiotics on composition of intestinal microflora of humans. Antimicrob Agents Chemother 31: 202–206
van der Waaij D 1989 The ecology of the human intestine and its consequences for overgrowth by pathogens such as Clostridium difficile. Annu Rev Microbiol 43: 69–87
Young VB, Schmidt TM 2004 Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol 42: 1203–1206
Gibson GR, Roberfroid MB 1995 Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr 125: 1401–1412
Scholz-Ahrens KE, Schaafsma G, van den Heuvel EG, Schrezenmeir J 2001 Effects of prebiotics on mineral metabolism. Am J Clin Nutr 73: 459S–464S
European Food Safety Authority (EFSA). Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission relating to the safety and suitability for particular nutritional use by infants of fructooligosaccharides in infant formulas and follow-on formulae. (Request no. EFSA-Q-2003-020). http://www.efsa.eu.int/science/nda/nda_opinions/226/opinion_nda_03_en1.pdf.
Hogenauer C, Hammer HF, Krejs GJ, Reisinger EC 1998 Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis 27: 702–710
Sullivan A, Edlund C, Nord CE 2001 Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 1: 101–114
Alverdy JC, Laughlin R, Wu L 2003 Influence of the critically ill state on host-pathogen interactions within the intestine: gut derived sepsis redefined. Crit Care Med 31: 598–607
Moubareck C, Gavini F, Vaugien L, Butel MJ, Doucet-Populaire F 2005 Antimicrobial susceptibility of bifidobacteria. J Antimicrob Chemother 55: 38–44
Yazid AM, Ali AM, Shuhaimi M, Kalaivaani V, Rokiah MY, Reezal A 2000 Antimicrobial susceptibility of bifidobacteria. Lett Appl Microbiol 31: 57–62
Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S 1992 Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37: 121–128
Madden JA, Plummer SF, Tang J, Garaiova I, Plummer NT, Herbison M, Hunter JO, Shimada T, Cheng L, Shirakawa T 2005 Effect of probiotics on preventing disruption of the intestinal microflora following antibiotic therapy: a double-blind, placebo-controlled pilot study. Int Immunopharmacol 5: 1091–1097
Figueroa G, Galeno H, Troncoso M, Toledo S, Soto V 1989 Prospective study of Campylobacter jejuni infection in Chilean infants evaluated by culture and serology. J Clin Microbiol 27: 1040–1044
Spencer E, Araya M, Sandino AM, Pacheco I, Brunser O 1988 Faecal excretion of rotavirus and other enteropathogens in newborns of the high and low socio-economic stratum in Santiago, Chile. Epidemiol Infect 101: 425–436
Acknowledgements
The authors thank M. M. Gonzalez, M.D., resident physician at the Health Center, M. Figueroa, R.N., and P. Mondaca, R.N., for help with the follow-up of children in the Field Station and sample collection. P. Torres is thanked for her excellent technical assistance in stool sample processing for FISH and R. Montalva for the flow-cytometry analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brunser, O., Gotteland, M., Cruchet, S. et al. Effect of a Milk Formula With Prebiotics on the Intestinal Microbiota of Infants After an Antibiotic Treatment. Pediatr Res 59, 451–456 (2006). https://doi.org/10.1203/01.pdr.0000198773.40937.61
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000198773.40937.61
This article is cited by
-
Oligosaccharide equine feed supplement, Immulix, has minor impact on vaccine responses in mice
Scientific Reports (2022)
-
Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants
Microbiome (2018)
-
The bifidogenic effect of inulin and oligofructose and its consequences for gut health
European Journal of Clinical Nutrition (2009)