Abstract
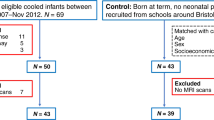
Magnetic resonance imaging studies have contributed to recognize the patterns of cerebral injury related to neonatal encephalopathy (NE). We assessed whether a smaller corpus callosum (CC) explained the difference in motor performance between school-age children with NE and controls. Frontal, middle, and posterior areas of the CC were measured in 61 9–10-y-old children with NE and in 47 controls. Motor performance was determined using the Movement Assessment Battery for Children (M-ABC). Linear regression was used to assess whether differences in M-ABC between NE children and controls could be explained by CC size. The CC of 11/30 children with NE type I according to Sarnat (NE I) and 19/36 children with NE type II according to Sarnat (NE II) showed generalized or focal thinning, compared with 8/49 controls. Children with NE II had significantly smaller middle and posterior parts and total areas of the CC. Children with NE scored significantly worse on the M-ABC than controls. The reduction in size of the posterior part of the CC partly explained the mean differences on the M-ABC. Children with NE have poorer motor skills than controls, which is partly explained by a smaller size of the CC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CC:
-
corpus callosum
- M-ABC:
-
Movement Assessment Battery for Children
- MCS:
-
midsagittal cerebral size
- NE:
-
neonatal encephalopathy
- NE I:
-
mild neonatal encephalopathy
- NE II:
-
moderate neonatal encephalopathy
- TIS:
-
Total Impairment Score
References
Ferriero DM 2004 Neonatal brain injury. N Engl J Med 351: 1985–1995
Sarnat HB, Sarnat MS 1976 Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 33: 696–705
Marlow N, Rose AS, Rands CE, Draper ES 2005 Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed 90: F380–F387
Robertson CM, Finer NN 1993 Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol 20: 483–500
van Handel M, Swaab H, de Vries LS, Jongmans MJ 2007 Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr 166: 645–654
Gonzalez FF, Miller SP 2006 Does perinatal asphyxia impair cognitive function without cerebral palsy?. Arch Dis Child Fetal Neonatal Ed 91: F454–F459
Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, Cowan F, Dubowitz L 2002 Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics 33: 242–248
Belet N, Belet U, Incesu L, Uysal S, Ozinal S, Keskin T, Sunter AT, Kucukoduk S 2004 Hypoxic-ischemic encephalopathy: correlation of serial MRI and outcome. Pediatr Neurol 31: 267–274
Rutherford M, Ward P, Allsop J, Malamatentiou C, Counsell S 2005 Magnetic resonance imaging in neonatal encephalopathy. Early Hum Dev 81: 13–25
Sie LT, van der Knaap MS, Oosting J, de Vries LS, Lafeber HN, Valk J 2000 MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics 31: 128–136
Barkovich AJ, Norman D 1988 Anomalies of the corpus callosum: correlation with further anomalies of the brain. AJR Am J Roentgenol 151: 171–179
Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A 1993 When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol 34: 71–75
Barkovich AJ, Kjos BO 1988 Normal postnatal development of the corpus callosum as demonstrated by MR imaging. AJNR Am J Neuroradiol 9: 487–491
Santhouse AM, Ffytche DH, Howard RJ, Williams SC, Stewart AL, Rooney M, Wyatt JS, Rifkin L, Murray RM 2002 The functional significance of perinatal corpus callosum damage: an fMRI study in young adults. Brain 125: 1782–1792
de Lacoste MC, Kirkpatrick JB, Ross ED 1985 Topography of the human corpus callosum. J Neuropathol Exp Neurol 44: 578–591
Rademaker KJ, Lam JN, Van Haastert IC, Uiterwaal CS, Lieftink AF, Groenendaal F, Grobbee DE, de Vries LS 2004 Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol 28: 279–287
Iai M, Tanabe Y, Goto M, Sugita K, Niimi H 1994 A comparative magnetic resonance imaging study of the corpus callosum in neurologically normal children and children with spastic diplegia. Acta Paediatr 83: 1086–1090
Mercuri E, Jongmans M, Henderson S, Pennock J, Chung YL, de Vries L, Dubowitz L 1996 Evaluation of the corpus callosum in clumsy children born prematurely: a functional and morphological study. Neuropediatrics 27: 317–322
Maneru C, Junque C, Salgado-Pineda P, Serra-Grabulosa JM, Bartres-Faz D, Ramirez-Ruiz B, Bargallo N, Tallada M, Botet F 2003 Corpus callosum atrophy in adolescents with antecedents of moderate perinatal asphyxia. Brain Inj 17: 1003–1009
Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS 2003 Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361: 736–742
Whalley HC, Wardlaw JM 2001 Accuracy and reproducibility of simple cross-sectional linear and area measurements of brain structures and their comparison with volume measurements. Neuroradiology 43: 263–271
Rauch RA, Jinkins JR 1994 Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res 64: 65–78
Henderson SE, Sugden DA, Smits-Engelsman BC 1998 Specifieke instructies voor de afname van de Movement ABC test. Movement Assessment Battery for Children. Swets & Zeitlinger, Lisse, Netherlands, pp 67–85
Witelson SF 1989 Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112: 799–835
Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM 2004 Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127: 2080–2089
Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, Glidden DV, Deming D, Partridge JC, Wu YW, Ashwal S, Ferriero DM 2005 Patterns of brain injury in term neonatal encephalopathy. J Pediatr 146: 453–460
Cordes I, Roland EH, Lupton BA, Hill A 1994 Early prediction of the development of microcephaly after hypoxic-ischemic encephalopathy in the full-term newborn. Pediatrics 93: 703–707
Mercuri E, Ricci D, Cowan FM, Lessing D, Frisone MF, Haataja L, Counsell SJ, Dubowitz LM, Rutherford MA 2000 Head growth in infants with hypoxic-ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics 106: 235–243
Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN 2004 Critical periods of brain growth and cognitive function in children. Brain 127: 321–329
Bishop KM, Wahlsten D 1997 Sex differences in the human corpus callosum: myth or reality?. Neurosci Biobehav Rev 21: 581–601
Ozmert EN, Yurdakok K, Soysal S, Kulak-Kayikci ME, Belgin E, Ozmert E, Laleli Y, Saracbasi O 2005 Relationship between physical, environmental and sociodemographic factors and school performance in primary schoolchildren. J Trop Pediatr 51: 25–32
Resnick MB, Gueorguieva RV, Carter RL, Ariet M, Sun Y, Roth J, Bucciarelli RL, Curran JS, Mahan CS 1999 The impact of low birth weight, perinatal conditions, and sociodemographic factors on educational outcome in kindergarten. Pediatrics 104: e74
Miyahara M, Piek J, Barrett N 2006 Accuracy of drawing in a dual-task and resistance-to-distraction study: motor or attention deficit?. Hum Mov Sci 25: 100–109
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Kooij, B., van Handel, M., Uiterwaal, C. et al. Corpus Callosum Size in Relation to Motor Performance in 9- to 10-Year-Old Children with Neonatal Encephalopathy. Pediatr Res 63, 103–108 (2008). https://doi.org/10.1203/PDR.0b013e31815b4435
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e31815b4435
This article is cited by
-
Comparison of MRI and neurosonogram 1- and 2-dimensional morphological measurements of the newborn corpus callosum
Pediatric Research (2019)
-
The long-term effect of perinatal asphyxia on hippocampal volumes
Pediatric Research (2019)
-
Neurodevelopmental outcome in survivors of hypoxic ischemic encephalopathy without cerebral palsy
European Journal of Pediatrics (2018)
-
Biometry of the corpus callosum assessed by 3D ultrasound and its correlation to neurodevelopmental outcome in very low birth weight infants
Journal of Perinatology (2017)