Abstract
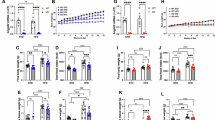
IUGR has been linked to the development of type 2 diabetes. Recent data suggest that some of the molecular defects underlying type 2 diabetes reside in the CNS. Disruption of the signal transducer and activator of transcription 3 (STAT3) in the hypothalamic neurons expressing leptin receptor, results in severe obesity, hyperglycaemia, and hyperinsulinemia. Our aim was to investigate the expression of STAT3 and its downstream effector proopiomelanocortin (POMC) in IUGR rats obtained by uterine artery ligation. On day 19 of gestation, time-dated Sprague-Dawley pregnant rats were anesthetized, and both the uterine arteries were ligated. At birth, hypothalamus was dissected and processed to evaluate the expression of STAT3, its phosphorylated form, and POMC. STAT3 mRNA, STAT3 protein, phosphorylated STAT3, POMC mRNA, and POMC protein were significantly reduced in IUGR versus sham animals (p < 0.0001, p < 0.05 and p < 0.001, p < 0.01, p < 0.01, respectively). No significant differences either in serum leptin concentrations or in hypothalamic leptin receptor expression were observed. Our results suggest that an abnormal intrauterine milieu can affect the hypothalamic expression of STAT3 and POMC at birth, altering the hypothalamic signaling pathways that regulate the energy homeostasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- POMC:
-
proopiomelanocortin
- STAT3:
-
signal transducer and activator of transcription 3
References
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ 1989 Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME 1989 Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM 1993 Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67
Barker DJ 1995 Fetal origins of coronary heart disease. BMJ 311: 171–174
Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ 2001 Early growth and coronary heart disease in later life: a longitudinal study. BMJ 322: 949–953
Geremia C, Cianfarani S 2004 Insulin sensitivity in children born small for gestational age (SGA). Rev Diabet Stud 1: 58–65
Fowden AL, Giussani DA, Forhead AJ 2005 Endocrine and metabolic programming during intrauterine development. Early Hum Dev 81: 723–734
Unterman T, Lascon R, Gotway M, Oehler D, Gounis A, Simmons RA, Ogata ES 1990 Circulating levels of insulin-like growth factor binding protein-1 (IGFBP-1) and hepatic mRNA are increased in the small for gestational age fetal rat. Endocrinology 127: 2035–2037
Simmons RA, Templeton LJ, Gertz SJ 2001 Intrauterine growth retardation leads to the development of type 2 diabetes in rat. Diabetes 50: 2279–2286
Puglianiello A, Cianfarani S 2006 Central control of glucose homeostasis. Rev Diabet Stud 3: 54–60
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L 2002 Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275
Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382
Elmquist JK, Marcus JN 2003 Rethinking the central causes of diabetes. Nat Med 9: 645–647
Prodi E, Obici S 2006 The brain as a molecular target for diabetic therapy. Endocrinology 147: 2664–2669
Lim CP, Cao X 2006 Structure, function, and regulation of STAT proteins. Mol Biosyst 2: 536–550
Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY 2004 Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101: 4661–4666
Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C 2004 Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24: 258–269
Gorogawa S, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, Takeda K, Akira S, Magnuson MA, Yamasaki Y, Kajimoto Y, Hori M 2004 Insulin secretory defects and impaired islet architecture in pancreatic beta-cell-specific STAT3 knockout mice. Biochem Biophys Res Commun 319: 1159–1170
Lee JY, Hennighausen L 2005 The transcription factor STAT3 is dispensable for pancreatic beta-cell development and function. Biochem Biophys Res Commun 334: 764–768
Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC 1996 Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93: 6231–6235
Darnell JE 1996 Reflections on STAT3, STAT5, and STAT6 as fat STATs. Proc Natl Acad Sci USA 93: 6221–6224
Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97
Myers MG, Cowley MA, Münzberg H 2008 Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556
Simmons RA, Gounis AS, Bangalore SA, Ogata ES 1992 Intrauterine growth retardation: fetal glucose transport is diminished in lung but spared in brain. Pediatr Res 31: 59–63
Puglianiello A, Germani D, Antignani S, Scalia Tomba G, Cianfarani S 2007 Changes in the expression of hypothalamic lipid sensing genes in rat model of intrauterine growth retardation (IUGR). Pediatr Res 61: 433–437
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443: 289–295
Gluckman PD, Hanson MA 2004 Living with the past: evolution, development, and patterns of disease. Science 305: 1733–1736
Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD 1997 Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165–168
Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Roger Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through aneural network in the arcuate nucleus. Nature 411: 480–484
Smith JT, Waddell BJ 2003 Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation nut is reduced by excess glucocorticoids. Endocrinology 144: 3024–3030
Desai M, Gayle D, Han G, Ross MG 2007 Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci 14: 329–337
Author information
Authors and Affiliations
Corresponding author
Additional information
No financial assistance was received to support this study.
Rights and permissions
About this article
Cite this article
Puglianiello, A., Germani, D. & Cianfarani, S. Exposure to Uteroplacental Insufficiency Reduces the Expression of Signal Transducer and Activator of Transcription 3 and Proopiomelanocortin in the Hypothalamus of Newborn Rats. Pediatr Res 66, 208–211 (2009). https://doi.org/10.1203/PDR.0b013e3181a9e7fd
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a9e7fd
This article is cited by
-
Effect of intrauterine growth retardation on liver and long-term metabolic risk
International Journal of Obesity (2012)
-
Mechanisms affecting neuroendocrine and epigenetic regulation of body weight and onset of puberty: Potential implications in the child born small for gestational age (SGA)
Reviews in Endocrine and Metabolic Disorders (2012)