Abstract
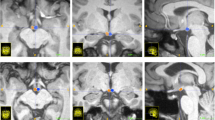
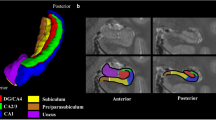
Congenital central hypoventilation syndrome (CCHS) is accompanied by reduced ventilatory sensitivity to CO2 and O2, respiratory drive failure during sleep, impaired autonomic, fluid, and food absorption regulation, and affective and cognitive deficits, including memory deficiencies. The deficits likely derive from neural injury, reflected as structural damage and impaired functional brain responses to ventilatory and autonomic challenges. Brain structures playing essential memory roles, including the hippocampus and anterior thalamus, are damaged in CCHS. Other memory formation circuitry, the fornix and mammillary bodies, have not been evaluated. We collected two high-resolution T1-weighted image series from 14 CCHS and 31 control subjects, using a 3.0-Tesla magnetic resonance imaging scanner. Image series were averaged and reoriented to a standard template; areas containing the mammillary bodies and fornices were over sampled, and body volumes and fornix cross-sectional areas were calculated and compared between groups. Both left and right mammillary body volumes and fornix cross-sectional areas were significantly reduced in CCHS over control subjects, controlling for age, gender, and intracranial volume. Damage to these structures may contribute to memory deficiencies found in CCHS. Hypoxic processes, together with diminished neuroprotection from micronutrient deficiencies secondary to fluid and dietary absorption issues, may contribute to the injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CCHS:
-
congenital central hypoventilation syndrome
- MB:
-
mammillary body
- MANCOVA:
-
multivariate analysis of covariance
- TIV:
-
total intracranial volume
References
1999 Idiopathic congenital central hypoventilation syndrome: diagnosis and management. American Thoracic Society. Am J Respir Crit Care Med 160: 368–373
Haddad GG, Mazza NM, Defendini R, Blanc WA, Driscoll JM, Epstein MA, Epstein RA, Mellins RB 1978 Congenital failure of automatic control of ventilation, gastrointestinal motility and heart rate. Medicine (Baltimore) 57: 517–526
Paton JY, Swaminathan S, Sargent CW, Keens TG 1989 Hypoxic and hypercapnic ventilatory responses in awake children with congenital central hypoventilation syndrome. Am Rev Respir Dis 140: 368–372
Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D 2004 Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol 37: 217–229
Ruof H, Hammer J, Tillmann B, Ghelfi D, Weber P 2008 Neuropsychological, behavioral, and adaptive functioning of Swiss children with congenital central hypoventilation syndrome. J Child Neurol 23: 1254–1259
Kumar R, Macey PM, Woo MA, Alger JR, Harper RM 2006 Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging 24: 1252–1258
Kumar R, Macey PM, Woo MA, Alger JR, Harper RM 2008 Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res 64: 275–280
Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM 2005 Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol 487: 361–371
Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR 2005 Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol 93: 1647–1658
Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM 2005 Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol 98: 958–969
Woo MA, Macey PM, Macey KE, Keens TG, Woo MS, Harper RK, Harper RM 2005 FMRI responses to hyperoxia in congenital central hypoventilation syndrome. Pediatr Res 57: 510–518
Macey PM, Macey KE, Woo MA, Keens TG, Harper RM 2005 Aberrant neural responses to cold pressor challenges in congenital central hypoventilation syndrome. Pediatr Res 57: 500–509
Lavenex PB, Amaral DG, Lavenex P 2006 Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci 26: 4546–4558
Ridley RM, Baker HF, Mills DA, Green ME, Cummings RM 2004 Topographical memory impairments after unilateral lesions of the anterior thalamus and contralateral inferotemporal cortex. Neuropsychologia 42: 1178–1191
Aggleton JP, Brown MW 1999 Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22: 425–444
Aggleton JP, Vann SD, Saunders RC 2005 Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci 22: 2519–2530
Shibata H 1992 Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. J Comp Neurol 323: 117–127
Victor M, Adams RD, Collins GH 1989 >The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd ed. pp FA Davis, Philadelphia
Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC 2006 The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res 147: 93–103
Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM 2008 Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett 438: 330–334
Kumar R, Woo MA, Birrer BV, Macey PM, Fonarow GC, Hamilton MA, Harper RM 2009 Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis 33: 236–242
Harper C 2009 The neuropathology of alcohol-related brain damage. Alcohol Alcohol 44: 136–140
Rorden C, Karnath HO, Bonilha L 2007 Improving lesion-symptom mapping. J Cogn Neurosci 19: 1081–1088
Ashburner J, Friston KJ 2005 Unified segmentation. Neuroimage 26: 839–851
Holmes EJ, Jacobson S, Stein BM, Butters N 1983 Ablations of the mammillary nuclei in monkeys: effects on postoperative memory. Exp Neurol 81: 97–113
Irle E, Markowitsch HJ 1982 Single and combined lesions of the cats thalamic mediodorsal nucleus and the mamillary bodies lead to severe deficits in the acquisition of an alternation task. Behav Brain Res 6: 147–165
Rosenstock J, Field TD, Greene E 1977 The role of mammillary bodies in spatial memory. Exp Neurol 55: 340–352
Vann SD, Aggleton JP 2003 Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract. J Neurosci 23: 3506–3514
Whishaw IQ, Maaswinkel H 1998 Rats with fimbria-fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction.”. J Neurosci 18: 3050–3058
Blair HT, Sharp PE 1995 Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J Neurosci 15: 6260–6270
Leutgeb S, Ragozzino KE, Mizumori SJ 2000 Convergence of head direction and place information in the CA1 region of hippocampus. Neuroscience 100: 11–19
Sharp PE, Tinkelman A, Cho J 2001 Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci 115: 571–588
Taube JS 1998 Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55: 225–256
Blair HT, Cho J, Sharp PE 1998 Role of the lateral mammillary nucleus in the rat head direction circuit: a combined single unit recording and lesion study. Neuron 21: 1387–1397
Conejo NM, Gonzalez-Pardo H, Vallejo G, Arias JL 2004 Involvement of the mammillary bodies in spatial working memory revealed by cytochrome oxidase activity. Brain Res 1011: 107–114
Bland BH, Konopacki J, Kirk IJ, Oddie SD, Dickson CT 1995 Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethan-anesthetized rat. J Neurophysiol 74: 322–333
Kirk IJ, Oddie SD, Konopacki J, Bland BH 1996 Evidence for differential control of posterior hypothalamic, supramammillary, and medial mammillary theta-related cellular discharge by ascending and descending pathways. J Neurosci 16: 5547–5554
Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC 1992 Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732
Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A 2004 Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201
Zhan G, Fenik P, Pratico D, Veasey SC 2005 Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med 171: 1414–1420
Gozal D, Daniel JM, Dohanich GP 2001 Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450
Pae EK, Chien P, Harper RM 2005 Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett 375: 123–128
Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O'Hearn E, Molliver ME, Aicher SA 2002 Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol 89: 331–359
Ang CW, Carlson GC, Coulter DA 2006 Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci 26: 11850–11856
Harper C 2006 Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe!. Eur J Neurol 13: 1078–1082
Kornreich L, Bron-Harlev E, Hoffmann C, Schwarz M, Konen O, Schoenfeld T, Straussberg R, Nahum E, Ibrahim AK, Eshel G, Horev G 2005 Thiamine deficiency in infants: MR findings in the brain. AJNR Am J Neuroradiol 26: 1668–1674
Read DJ 1978 The aetiology of the sudden infant death syndrome: current ideas on breathing and sleep and possible links to deranged thiamine neurochemistry. Aust N Z J Med 8: 322–336
Singleton CK, Martin PR 2001 Molecular mechanisms of thiamine utilization. Curr Mol Med 1: 197–207
Shin BH, Choi SH, Cho EY, Shin MJ, Hwang KC, Cho HK, Chung JH, Jang Y 2004 Thiamine attenuates hypoxia-induced cell death in cultured neonatal rat cardiomyocytes. Mol Cells 18: 133–140
Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S 2003 Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33: 459–461
Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM 2008 Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol 164: 38–48
Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF 2003 Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130: 6635–6642
Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C 2008 A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072
Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG 2006 Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314
Onimaru H, Ikeda K, Kawakami K 2008 CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28: 12845–12850
Sullivan EV, Lane B, Deshmukh A, Rosenbloom MJ, Desmond JE, Lim KO, Pfefferbaum A 1999 In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res 23: 1629–1636
Acknowledgements
We thank Ms. Rebecca Harper and Ms. Annaise Magliore for assistance with data collection, and parents and children for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Institute of Child Health and Human Development R01 HD-22695.
Rights and permissions
About this article
Cite this article
Kumar, R., Lee, K., Macey, P. et al. Mammillary Body and Fornix Injury in Congenital Central Hypoventilation Syndrome. Pediatr Res 66, 429–434 (2009). https://doi.org/10.1203/PDR.0b013e3181b3b363
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b3b363
This article is cited by
-
Neurodevelopmental outcome and respiratory management of congenital central hypoventilation syndrome: a retrospective study
BMC Pediatrics (2020)
-
Reduced brain mammillary body volumes and memory deficits in adolescents who have undergone the Fontan procedure
Pediatric Research (2020)
-
Progressive gray matter changes in patients with congenital central hypoventilation syndrome
Pediatric Research (2012)