Abstract
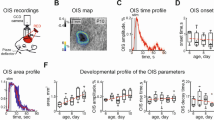
We investigated the extent of isoflurane-induced neurodegeneration on the fetuses of pregnant rats exposed in utero. Pregnant rats at gestational d 21 were divided into three experimental groups. Rats in the control group spontaneously breathed 100% oxygen for 1 h. Rats in the treatment groups breathed either 1.3 or 3% isoflurane in 100% oxygen through an endotracheal tube, with mechanical ventilation for 1 h. Rat pups were delivered by cesarian section 6 h after treatment, and fetal blood was sampled from the left ventricle of each fetal heart and evaluated for S100β. Fetal brains were then evaluated for apoptosis, using caspase-3 immunohistochemistry in the CA1 region of the hippocampus and the retrosplenial cortex (RS). The 3% isoflurane treatment group showed significantly higher levels of S100β levels and significantly increased average densities of total caspase-3–positive cells in the CA1 hippocampus and RS cortex compared with the control and the 1.3% isoflurane groups. There were no differences in S100β levels or densities of caspase-3–positive cells between the control and 1.3% isoflurane groups. Isoflurane at a concentration of 3% for 1 h increased neurodegeneration in the hippocampal CA1 area and the retrosplenial cortex in the developing brain of fetal rats.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ABG:
-
arterial blood gas
- MAC:
-
minimal alveolar concentration
- MAP:
-
mean arterial blood pressure
References
Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS 2005 Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg 101: 651–657
Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff R 2008 The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108: 251–260
Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF 2008 Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109: 243–250
Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE 2007 The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci 27: 1247–1254
Malledant Y, Siproudhis L, Tanguy M, Clerc C, Chesne C, Saint-Marc C, Guillouzo A 1990 Effects of halothane on human and rat hepatocyte cultures. Anesthesiology 72: 526–534
Chang YC, Chou MY 2001 Cytotoxicity of halothane on human gingival fibroblast cultures in vitro. J Endod 27: 82–84
Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG 2005 Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 1037: 139–147
Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF 2008 A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg 106: 492–500
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF 2003 Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23: 876–882
Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M 2007 Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 106: 746–753
Culley DJ, Baxter MG, Yukhananov R, Crosby G 2004 Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100: 309–314
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO 2009 Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110: 796–804
Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP 2009 Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110: 805–812
Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V 2008 Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol 18: 198–210
Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H 2007 Effect of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 53: 942–950
Myers LB, Cohen D, Galinkin J, Gaiser R, Kurth CD 2002 Anaesthesia for fetal surgery. Paediatr Anaesth 12: 569–578
Goldsmith MF, Straus SE, Kupfer C, Lenfant C, Collins F, Hodes RJ, Gordis E, Fauci AS, Alexander D, Battey JF, Slavkin HC, Leshner AI, Olden K, Hyman SE, Fischbach GD 1999 2020 vision: NIH heads foresee the future. JAMA 282: 2287–2290
Wei H, Liang G, Yang H 2007 Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett 425: 59–62
Dobbing J, Sands J 1979 Comparative aspects of the brain growth spurt. Early Hum Dev 3: 79–83
Ngan Kee WD, Khaw KS 2006 Vasopressors in obstetrics: what should we be using?. Curr Opin Anaesthesiol 19: 238–243
Michetti F, Gazzolo D 2003 S100B testing in pregnancy. Clin Chim Acta 335: 1–7
Kofke WA, Konitzer P, Meng QC, Guo J, Cheung A 2004 The effect of apolipoprotein E genotype on neuron specific enolase and S-100beta levels after cardiac surgery. Anesth Analg 99: 1323–1325
Paxinos G, Ashwell KW, Tork I 1995 Atlas of the Developing Rat Nervous System. 2nd Academic Press, San Diego
McClaine RJ, Uemura K, de la Fuente SG, Manson RJ, Booth JV, White WD, Campbell KA, McClaine DJ, Benni PB, Eubanks WS, Reynolds JD 2005 General anesthesia improves fetal cerebral oxygenation without evidence of subsequent neuronal injury. J Cereb Blood Flow Metab 25: 1060–1069
Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgansberger W, Ohl F, Kochs EF, Blobner M 2009 Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology 56: 626–636
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M 2007 Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106: 436–443
Acknowledgements
We gratefully recognize the discussions with Roderic Eckenhoff, M.D., Professor of Anesthesia, Maryellen Eckenhoff, Ph.D., Research Associate, and Randall Pittman, Ph.D., Professor of Pharmacology, from the University Of Pennsylvania School of Medicine, Philadelphia, PA. We thank Dr. Christopher Ward from the Children Hospital of Philadelphia (CHOP) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Peretich, K., Zhao, Y. et al. Anesthesia-Induced Neurodegeneration in Fetal Rat Brains. Pediatr Res 66, 435–440 (2009). https://doi.org/10.1203/PDR.0b013e3181b3381b
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b3381b
This article is cited by
-
Comparison of 2 anesthetic protocols and surgical timing during cesarean section on neonatal vitality and umbilical cord blood parameters
BMC Veterinary Research (2023)
-
A synthetic peptide rescues rat cortical neurons from anesthetic-induced cell death, perturbation of growth and synaptic assembly
Scientific Reports (2021)
-
Effects of Sevoflurane Exposure During Late Pregnancy on Brain Development and Beneficial Effects of Enriched Environment on Offspring Cognition
Cellular and Molecular Neurobiology (2020)
-
Effects of short-term exposure to sevoflurane on the survival, proliferation, apoptosis, and differentiation of neural precursor cells derived from human embryonic stem cells
Journal of Anesthesia (2017)
-
Propofol administration to the maternal-fetal unit improved fetal EEG and influenced cerebral apoptotic pathway in preterm lambs suffering from severe asphyxia
Molecular and Cellular Pediatrics (2015)