Abstract
Introduction:
To implement neuroprotective strategies in newborns, sensitive and specific biomarkers are needed for identifying those who are at risk for brain damage. We evaluated the effectiveness of matrix metalloproteinases (MMPs) and their naturally occurring tissue inhibitors of metalloproteinases (TIMPs) in predicting neonatal encephalopathy (NE) damage in newborns.
Results:
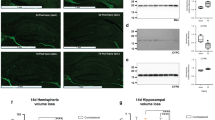
Plasma MMP-9 and TIMP-1 levels were upregulated as early as 1 h after the HI insult but not did not show such elevations after other types of injury (ibotenate-induced excitotoxicity, hypoxia, lipopolysaccharide-induced inflammation), and brain levels reflected this increase soon thereafter. We confirmed these results by carrying out plasma MMP-9 and TIMP-1 measurements in human newborns with NE. In these infants, protein levels of MMP-9 and TIMP-1 were found to be elevated during a short window up to 6 h after birth.
Discussion:
This feature is particularly useful in identifying newborns in need of neuroprotection. A second peak observed 72 h after birth is possibly related to the second phase of energy failure after a HI insult. Our data, although preliminary, support the use of MMP-9 and TIMP-1 as early biomarkers for the presence and extent of perinatal brain injury in human term newborns.
Methods:
We first used a mouse model of neonatal HI injury to explore mechanistic aspects such as the time course of these markers after the hypoxia–ischemia event, and the correlation between the levels of these candidate markers in brain and plasma.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Okereafor A, Allsop J, Counsell SJ, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008;121: 906–14.
Bodensteiner JB, Johnsen SD . Magnetic resonance imaging (MRI) findings in children surviving extremely premature delivery and extremely low birthweight with cerebral palsy. J Child Neurol 2006;21: 743–7.
Dzwonek J, Rylski M, Kaczmarek L . Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett 2004;567: 129–35.
Ranasinghe HS, Williams CE, Christophidis LJ, Mitchell MD, Fraser M, Scheepens A . Proteolytic activity during cortical development is distinct from that involved in hypoxic ischemic injury. Neuroscience 2009;158: 732–44.
Agrawal SM, Lau L, Yong VW . MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol 2008;19: 42–51.
Wang X, Jung J, Asahi M, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci 2000;20: 7037–42.
Svedin P, Hagberg H, Sävman K, Zhu C, Mallard C . Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci 2007;27: 1511–8.
Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006;12: 441–5.
Cunningham LA, Wetzel M, Rosenberg GA . Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 2005;50: 329–39.
Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M . Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci 1997;17: 4223–35.
Magnoni S, Baker A, Thomson S, et al. Neuroprotective effect of adenoviral-mediated gene transfer of TIMP-1 and -2 in ischemic brain injury. Gene Ther 2007;14: 621–5.
Tejima E, Guo S, Murata Y, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma 2009;26: 1935–41.
Docherty AJ, O’Connell J, Crabbe T, Angal S, Murphy G . The matrix metalloproteinases and their natural inhibitors: prospects for treating degenerative tissue diseases. Trends Biotechnol 1992;10: 200–7.
Montaner J, Alvarez-Sabín J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke 2001;32: 1759–66.
Rice JE 3rd, Vannucci RC, Brierley JB . The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981;9: 131–41.
Marret S, Mukendi R, Gadisseux JF, Gressens P, Evrard P . Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol 1995;54: 358–70.
Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci 2001;13: 1101–6.
Medja F, Lelièvre V, Fontaine RH, et al. Thiorphan, a neutral endopeptidase inhibitor used for diarrhoea, is neuroprotective in newborn mice. Brain 2006;129(Pt 12): 3209–23.
Bednarek N, Clément Y, Lelièvre V, et al. Ontogeny of MMPs and TIMPs in the murine neocortex. Pediatr Res 2009;65: 296–300.
Volpe JJ . Neurology of the Newborn. Philadelphia, PA: Saunders, 2008: 401–80.
Chen W, Hartman R, Ayer R, et al. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem 2009;111: 726–36.
Tan HK, Heywood D, Ralph GS, Bienemann A, Baker AH, Uney JB . Tissue inhibitor of metalloproteinase 1 inhibits excitotoxic cell death in neurons. Mol Cell Neurosci 2003;22: 98–106.
Worthmann H, Tryc AB, Goldbecker A, et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis 2010;30: 85–92.
Hasegawa K, Ichiyama T, Isumi H, Nakata M, Sase M, Furukawa S . NF-kappaB activation in peripheral blood mononuclear cells in neonatal asphyxia. Clin Exp Immunol 2003;132: 261–4.
Tong W, Chen W, Ostrowski RP, et al. Maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPs in the brain of neonatal rats. Dev Neurobiol 2010;70: 182–94.
Dragun P, Makarewicz D, Wójcik L, Ziemka-Nalecz M, Slomka M, Zalewska T . Matrix metaloproteinases activity during the evolution of hypoxic-ischemic brain damage in the immature rat. The effect of 1-methylnicotinamide (MNA). J Physiol Pharmacol 2008;59: 441–55.
Lorek A, Takey Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the new born piglet: continuous 48 hours studies by phosphorus magnetic resonance spectroscopy. Ped Res 1994;36: 699–706.
Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107: 598–603.
Lorenzl S, De Pasquale G, Segal AZ, Beal MF . Dysregulation of the levels of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in the early phase of cerebral ischemia. Stroke 2003;34: e37–8; author reply e37–8.
Sunagawa S, Ichiyama T, Honda R, Fukunaga S, Maeba S, Furukawa S . Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in perinatal asphyxia. Brain Dev 2009;31: 588–93.
Sarnat HB, Sarnat MS . Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33: 696–705.
Acknowledgements
We thank Jorge Gallego (INSERM U676), Ryan Colvin (St Louis Children’s Hospital, Washington University, St Louis MO), and Damien Jolly (CHU Reims) for their help with the statistical analysis of the data; and Paul Toubas (State of University of New York, Downstate Medical Center, Brooklyn, New York), Pierre Desautels, and Amit Mathur (St Louis Children’s Hospital, Washington University, St Louis MO) for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no financial or other conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Bednarek, N., Svedin, P., Garnotel, R. et al. Increased MMP-9 and TIMP-1 in mouse neonatal brain and plasma and in human neonatal plasma after hypoxia–ischemia: a potential marker of neonatal encephalopathy. Pediatr Res 71, 63–70 (2012). https://doi.org/10.1038/pr.2011.3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2011.3
This article is cited by
-
Exploratory factor analysis yields grouping of brain injury biomarkers significantly associated with outcomes in neonatal and pediatric ECMO
Scientific Reports (2024)
-
LyTONEPAL: long term outcome of neonatal hypoxic encephalopathy in the era of neuroprotective treatment with hypothermia: a French population-based cohort
BMC Pediatrics (2018)
-
Loss of interneurons and disruption of perineuronal nets in the cerebral cortex following hypoxia-ischaemia in near-term fetal sheep
Scientific Reports (2018)
-
Postnatal LPS Challenge Impacts Escape Learning and Expression of Plasticity Factors Mmp9 and Timp1 in Rats: Effects of Repeated Training
Neurotoxicity Research (2017)
-
Expression of the FGF2 and TIMP1 Genes in the Adult Rat Brain after Administration of Interleukin-1β during Early Postnatal Ontogeny
Neuroscience and Behavioral Physiology (2016)