Abstract
Background:
Regression is an important process in the normal development of many organs. In this study, we investigated whether glomerular regression occurs after normal glomerulogenesis and determined the time course for this process.
Methods:
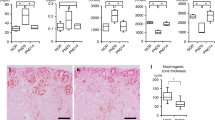
Glomerular number was analyzed in normal mouse kidneys at postnatal day (P)7, P10, P14, P18, P21, P25, and P28 by the gold standard fractionator/dissector method, which involves exhausting the kidney tissue. Vascular regression markers, angiopoietin 2 (ANGPT2), and thrombospondin 1 (THBS1), were examined by immunohistochemistry.
Results:
The maximum glomerular number was reached at P7 with 14,051 glomeruli per kidney (95% confidence interval: 12,084–16,018). This peak was followed by a progressive reduction, with a nadir of 11,060 (10,393–11,727) occurring at P18 (P < 0.05 as compared with P7). Thereafter, glomerular number remained constant. Complementary immunohistochemical examination of vascular regression markers showed peak expression of glomerular ANGPT2 and THBS1 at P14.
Conclusion:
Our study reveals that the tissue- and time-saving Weibel–Gomez method commonly used to assess glomerular number is valid only after P18. The data indicate that regulation of glomerular number by regression occurs in normally maturing mouse kidneys. These findings suggest that the process of glomerular regression could be therapeutically targeted to prevent oligonephronia, which otherwise predisposes to chronic kidney disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang H, Hu X, Tse J, Tilahun F, Qiu M, Chen L . Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci 2011;52:334–8.
Davidson AJ. Mouse kidney development. 2008. (http://www.ncbi.nlm.nih.gov/pubmed/20614633.)
Saint-Geniez M, D’Amore PA . Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol 2004;48:1045–58.
Keller G, Zimmer G, Mall G, Ritz E, Amann K . Nephron number in patients with primary hypertension. N Engl J Med 2003;348:101–8.
Zandi-Nejad K, Luyckx VA, Brenner BM . Adult hypertension and kidney disease: the role of fetal programming. Hypertension 2006;47:502–8.
Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE . Human nephron number: implications for health and disease. Pediatr Nephrol 2011;26:1529–33.
Larsson L, Aperia A, Wilton P . Effect of normal development on compensatory renal growth. Kidney Int 1980;18:29–35.
Tomat AL, Inserra F, Veiras L, et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol 2008; 295:R543–9.
Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM . Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 2007;18:1688–96.
Schreuder MF, Nyengaard JR, Remmers F, van Wijk JA, Delemarre-van de Waal HA . Postnatal food restriction in the rat as a model for a low nephron endowment. Am J Physiol Renal Physiol 2006;291:F1104–7.
Nyengaard JR . Stereologic methods and their application in kidney research. J Am Soc Nephrol 1999;10:1100–23.
Cullen-McEwen LA, Armitage JA, Nyengaard JR, Moritz KM, Bertram JF . A design-based method for estimating glomerular number in the developing kidney. Am J Physiol Renal Physiol 2011;300:F1448–53.
Weibel ER, Gomez DM . A principle for counting tissue structures on random sections. J Appl Physiol 1962;17:343–8.
Adamczak M, Gross ML, Amann K, Ritz E . Reversal of glomerular lesions involves coordinated restructuring of glomerular microvasculature. J Am Soc Nephrol 2004;15:3063–72.
Weibel ER . Numerical density: shape and size of particles. In: Weibel ER, ed. Stereological Methods Vol. 2: Theoretical Foundations. London: Academic Press, 1980:140–74.
Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF . Applicability of the glomerular size distribution coefficient in assessing human glomerular volume: the Weibel and Gomez method revisited. J Anat 2007;210:578–82.
Woolf AS . Angiopoietins: vascular growth factors looking for roles in glomeruli. Curr Opin Nephrol Hypertens 2010;19:20–5.
Augustin HG, Koh GY, Thurston G, Alitalo K . Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009;10:165–77.
Mirochnik Y, Kwiatek A, Volpert OV . Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets 2008;9:851–62.
Campbell N, Greenaway J, Henkin J, Petrik J . ABT-898 induces tumor regression and prolongs survival in a mouse model of epithelial ovarian cancer. Mol Cancer Ther 2011;10:1876–85.
Solomon S . Developmental changes in nephron number, proximal tubular length and superficial nephron glomerular filtration rate of rats. J Physiol (Lond) 1977;272:573–89.
Hartman HA, Lai HL, Patterson LT . Cessation of renal morphogenesis in mice. Dev Biol 2007;310:379–87.
Chevalier RL, Thornhill BA, Chang AY, Cachat F, Lackey A . Recovery from release of ureteral obstruction in the rat: relationship to nephrogenesis. Kidney Int 2002;61:2033–43.
Nyengaard JR . Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int 1993;43:1049–57.
De Spiegelaere W, Cornillie P, Simoens P, Van den Broeck W . Immunohistochemical detection of the angiopoietins during porcine metanephric kidney development. Acta Histochem 2011;113:585–90.
Yuan HT, Suri C, Landon DN, Yancopoulos GD, Woolf AS . Angiopoietin-2 is a site-specific factor in differentiation of mouse renal vasculature. J Am Soc Nephrol 2000;11:1055–66.
Yuan HT, Suri C, Yancopoulos GD, Woolf AS . Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J Am Soc Nephrol 1999;10:1722–36.
Davis B, Dei Cas A, Long DA,et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 2007;18:2320–9.
Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P . Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 1993;197:40–56.
Hugo C, Daniel C . Thrombospondin in renal disease. Nephron Exp Nephrol 2009;111:e61–6.
Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N . Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 2003;228:630–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, J., Perrien, D., Yang, HC. et al. Maturational regression of glomeruli determines the nephron population in normal mice. Pediatr Res 72, 241–248 (2012). https://doi.org/10.1038/pr.2012.81
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.81
This article is cited by
-
Cep55 promotes cytokinesis of neural progenitors but is dispensable for most mammalian cell divisions
Nature Communications (2020)
-
Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction
Kidney International (2016)