Abstract
Background:
The use of oxygen in acute treatment of asphyxiated term newborns is associated with increased mortality. It is unclear how hyperoxic reoxygenation after hypoxia affects transcriptional changes in the newborn lung.
Methods:
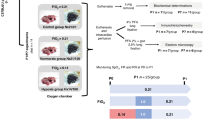
On postnatal day 7, C57BL/6 mice (n = 62) were randomized to 120-min hypoxia (fraction of inspired oxygen (FiO2) 0.08) or normoxia. The hypoxia group was further randomized to reoxygenation for 30 min with FiO2 0.21, 0.40, 0.60, or 1.00, and the normoxia group to FiO2 0.21 or 1.00. Transcriptome profiling was performed on homogenized lung tissue using the Affymetrix 750k expression array, and validation was carried out by real-time polymerase chain reaction and enzyme-linked immunosorbent assay.
Results:
The hypoxia–reoxygenation model induced hypoxia-inducible factor 1 (HIF-1) targets like Vegfc, Adm, and Aqp1. In total, ~70% of the significantly differentially expressed genes were detected in the two high hyperoxic groups (FiO2 0.60 and 1.00). Reoxygenation with 100% oxygen after hypoxia uniquely upregulated Gadd45g, Dusp1, Peg3, and Tgm2. Pathway analysis identified mammalian target of rapamycin (mTOR) signaling pathway, DNA repair, c-jun N-terminal kinase (JNK)-pathway regulation, and cell cycle after hyperoxic reoxygenation was applied.
Conclusion:
Acute hypoxia induces HIF-1 targets independent of the reoxygenation regime applied. Hyperoxic reoxygenation affects pathways regulating cell growth and survival. DNA-damage–responsive genes are restricted to reoxygenation with 100% oxygen.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA . Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol 2011;178:2601–10.
Saugstad OD . Is oxygen more toxic than currently believed? Pediatrics 2001;108:1203–5.
Saugstad OD, Ramji S, Soll RF, Vento M . Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008;94:176–82.
Kondo M, Itoh S, Isobe K, et al. Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during resuscitation periods after asphyxiation load. Pediatr Res 2000;47(4 Pt 1):524–7.
Solberg R, Andresen JH, Escrig R, Vento M, Saugstad OD . Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr Res 2007;62:559–63.
Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS . Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology 2009;95:299–305.
Farrow KN, Lee KJ, Perez M, et al. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal 2012;17:460–70.
O’Donovan DJ, Fernandes CJ . Free radicals and diseases in premature infants. Antioxid Redox Signal 2004;6:169–76.
Bhandari A, Bhandari V . Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–73.
Perlman JM, Wyllie J, Kattwinkel J, et al.; Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122:16 Suppl 2:S516–38.
Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124:e439–49.
Munkeby BH, Børke WB, Bjørnland K, et al. Resuscitation of hypoxic piglets with 100% O2 increases pulmonary metalloproteinases and IL-8. Pediatr Res 2005;58:542–8.
Wagenaar GT, ter Horst SA, van Gastelen MA, et al. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 2004;36:782–801.
Lee PJ, Choi AM . Pathways of cell signaling in hyperoxia. Free Radic Biol Med 2003;35:341–50.
Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA . Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 2003;29:779–83.
Ben-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A . Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res 2002;90:784–91.
Sejersted Y, Aasland AL, Bjørås M, Eide L, Saugstad OD . Accumulation of 8-oxoguanine in liver DNA during hyperoxic resuscitation of newborn mice. Pediatr Res 2009;66:533–8.
Truog WE, Xu D, Ekekezie II, et al. Chronic hypoxia and rat lung development: analysis by morphometry and directed microarray. Pediatr Res 2008;64:56–62.
Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J . Multiple organ involvement in perinatal asphyxia. J Pediatr 1995;127:786–93.
Low JA . Intrapartum fetal asphyxia: definition, diagnosis, and classification. Am J Obstet Gynecol 1997;176:957–9.
Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM . Postnatal growth of the mouse lung. J Anat 1977;124(Pt 1):131–51.
Heidecker B, Kasper EK, Wittstein IS, et al. Transcriptomic biomarkers for individual risk assessment in new-onset heart failure. Circulation 2008;118:238–46.
Gustavsson M, Wilson MA, Mallard C, Rousset C, Johnston MV, Hagberg H . Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res 2007;61:444–50.
Janér J, Lassus P, Haglund C, Paavonen K, Alitalo K, Andersson S . Pulmonary vascular endothelial growth factor-C in development and lung injury in preterm infants. Am J Respir Crit Care Med 2006;174:326–30.
Remesal A, Pedraz C, San Feliciano L, Ludeña D . Pulmonary expression of vascular endothelial growth factor (VEGF) and alveolar septation in a newborn rat model exposed to acute hypoxia and recovered under conditions of air or hyperoxia. Histol Histopathol 2009;24:325–30.
O’Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC . p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2000;278:L552–9.
Agorreta J, Zulueta JJ, Montuenga LM, Garayoa M . Adrenomedullin expression in a rat model of acute lung injury induced by hypoxia and LPS. Am J Physiol Lung Cell Mol Physiol 2005;288:L536–45.
Sciesielski LK, Paliege A, Martinka P, Scholz H . Enhanced pulmonary expression of the TrkB neurotrophin receptor in hypoxic rats is associated with increased acetylcholine-induced airway contractility. Acta Physiol (Oxf) 2009;197:253–64.
Abreu-Rodríguez I, Sánchez Silva R, Martins AP, et al. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1a. PLoS ONE 2011;6:e28385.
Cui LY, Yang S, Zhang J . Protective effects of neutrophil gelatinase-associated lipocalin on hypoxia/reoxygenation injury of HK-2 cells. Transplant Proc 2011;43:3622–7.
Roudkenar MH, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M . Neutrophil gelatinase-associated lipocalin: a new antioxidant that exerts its cytoprotective effect independent on Heme Oxygenase-1. Free Radic Res 2011;45:810–9.
Kumarasamy A, Schmitt I, Nave AH, et al. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med 2009;180:1239–52.
Feng Z, Levine AJ . The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 2010;20:427–34.
Ahmad A, Ahmad S, Chang LY, Schaack J, White CW . Endothelial Akt activation by hyperoxia: role in cell survival. Free Radic Biol Med 2006;40:1108–18.
Carnesecchi S, Deffert C, Pagano A, et al. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 2009;180:972–81.
Houtgraaf JH, Versmissen J, van der Giessen WJ . A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med 2006;7:165–72.
Randerath E, Zhou GD, Randerath K . Organ-specific oxidative DNA damage associated with normal birth in rats. Carcinogenesis 1997;18:859–66.
Rancourt RC, Keng PC, Helt CE, O’Reilly MA . The role of p21(CIP1/WAF1) in growth of epithelial cells exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2001;280:L617–26.
Dalen ML, Alme TN, Bjørås M, Munkeby BH, Rootwelt T, Saugstad OD . Reduced expression of DNA glycosylases in post-hypoxic newborn pigs undergoing therapeutic hypothermia. Brain Res 2010;1363:198–205.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8.
Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–64.
Ishwaran H, Rao JS, Kogalur UB . BAMarraytrade mark: Java software for Bayesian analysis of variance for microarray data. BMC Bioinformatics 2006;7:59.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50.
Edgar R, Domrachev M, Lash AE . Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 2002;30:207–10.
Acknowledgements
We express gratitude to Monica Atneosen-Aasegg and Grethe Dyrhaug for their assistance during the animal experiments and RT-PCR analysis. We also thank Grethe Dyrhaug and Maren Bakkebø for their contribution with the protein analysis. The animal experiments were performed at the Centre of Comparative Medicine, Oslo University Hospital, Rikshospitalet, and we appreciate the help and facilities that we were offered during our work.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure and Tables.
(ZIP 1828 kb)
Rights and permissions
About this article
Cite this article
Wollen, E., Sejersted, Y., Wright, M. et al. Transcriptome profiling of the newborn mouse lung after hypoxia and reoxygenation: hyperoxic reoxygenation affects mTOR signaling pathway, DNA repair, and JNK-pathway regulation. Pediatr Res 74, 536–544 (2013). https://doi.org/10.1038/pr.2013.140
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.140
This article is cited by
-
Neonatal chest compressions: time to act
Pediatric Research (2021)
-
Short-term perinatal oxygen exposure may impair lung development in adult mice
Biological Research (2020)
-
Oxygen therapy of the newborn from molecular understanding to clinical practice
Pediatric Research (2019)
-
The fetal circulation, pathophysiology of hypoxemic respiratory failure and pulmonary hypertension in neonates, and the role of oxygen therapy
Journal of Perinatology (2016)
-
Increased expression of inflammatory genes in the neonatal mouse brain after hyperoxic reoxygenation
Pediatric Research (2015)