Abstract
Background:
Bronchopulmonary dysplasia (BPD) is associated with perinatal inflammatory triggers. Methods targeting bacterial rRNA may improve detection of microbial colonization in premature infants. We hypothesize that respiratory microbiota differs between preterm infants who develop BPD and those unaffected and correlates with inflammatory mediator concentrations.
Methods:
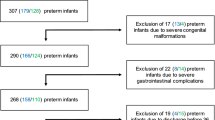
Twenty-five infants, born at ≤32 wk of gestation and intubated in the first 24 h, were enrolled. Tracheal aspirates were obtained at intubation and on days 3, 7, and 28. Bacterial DNA was extracted, and 16S rRNA genes were amplified and sequenced. Concentrations of interleukins (IL-1β, IL-6, IL-8, IL-10, and IL-12), tumor necrosis factor-α, interferon-γ, lipopolysaccharide (LPS), and lipoteichoic acid (LTA) were measured. Chorioamnionitis was diagnosed by histology. BPD was defined as an oxygen requirement at 36 wk postmenstrual age.
Results:
Acinetobacter was the predominant genus in the airways of all infants at birth. Ten infants developed BPD and showed reduced bacterial diversity at birth. No differences were detected in bacterial diversity, cytokines, LPS, and LTA from infants with and without exposure to chorioamnionitis.
Conclusion:
The airways of premature infants are not sterile at birth. Reduced diversity of the microbiome may be an important factor in the development of BPD and is not associated with differences in inflammatory mediators.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fanaroff AA, Stoll BJ, Wright LL, et al.; NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007;196:147.e1–8.
Stoll BJ, Hansen NI, Bell EF, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–56.
Thome UH, Ambalavanan N . Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med 2009;14:21–7.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9.
Ehrenkranz RA, Walsh MC, Vohr BR, et al.; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116:1353–60.
Bancalari E . Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol 2001;18:1–9.
Coalson JJ . Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8:73–81.
Kramer BW, Kallapur S, Newnham J, Jobe AH . Prenatal inflammation and lung development. Semin Fetal Neonatal Med 2009;14:2–7.
Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 2004;55:1009–17.
D’Angio CT, Basavegowda K, Avissar NE, Finkelstein JN, Sinkin RA . Comparison of tracheal aspirate and bronchoalveolar lavage specimens from premature infants. Biol Neonate 2002;82:145–9.
Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE . Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:155–61.
Watterberg KL, Demers LM, Scott SM, Murphy S . Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–5.
Jobe AH, Kallapur SG . Chorioamnionitis, surfactant, and lung disease in very low birth weight infants. J Pediatr 2010;156:3–4.
Van Marter LJ, Dammann O, Allred EN, et al.; Developmental Epidemiology Network Investigators. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002;140:171–6.
Beeton ML, Maxwell NC, Davies PL, et al. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur Respir J 2011;37:1424–30.
Cordero L, Ayers LW, Davis K . Neonatal airway colonization with gram-negative bacilli: association with severity of bronchopulmonary dysplasia. Pediatr Infect Dis J 1997;16:18–23.
Schelonka RL, Waites KB . Ureaplasma infection and neonatal lung disease. Semin Perinatol 2007;31:2–9.
Couroucli XI, Welty SE, Ramsay PL, et al. Detection of microorganisms in the tracheal aspirates of preterm infants by polymerase chain reaction: association of adenovirus infection with bronchopulmonary dysplasia. Pediatr Res 2000;47:225–32.
Ballard HO, Shook LA, Bernard P, et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr Pulmonol 2011;46:111–8.
Rogers GB, Carroll MP, Bruce KD . Studying bacterial infections through culture-independent approaches. J Med Microbiol 2009;58(Pt 11):1401–18.
Rogers GB, Daniels TW, Tuck A, et al. Studying bacteria in respiratory specimens by using conventional and molecular microbiological approaches. BMC Pulm Med 2009;9:14.
Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J . Metagenomic pyrosequencing and microbial identification. Clin Chem 2009;55:856–66.
Hamady M, Knight R . Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res 2009;19:1141–52.
Hattori M, Taylor TD . The human intestinal microbiome: a new frontier of human biology. DNA Res 2009;16:1–12.
Nasution TA, Cheong SF, Lim CT, Leong EW, Ngeow YF . Multiplex PCR for the detection of urogenital pathogens in mothers and newborns. Malays J Pathol 2007;29:19–24.
Stressmann FA, Connett GJ, Goss K, et al. The use of culture-independent tools to characterize bacteria in endo-tracheal aspirates from pre-term infants at risk of bronchopulmonary dysplasia. J Perinat Med 2010;38:333–7.
Mourani PM, Harris JK, Sontag MK, Robertson CE, Abman SH . Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS One 2011;6:e25959.
DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056.
Nathe KE, Parad R, Van Marter LJ, et al. Endotoxin-directed innate immunity in tracheal aspirates of mechanically ventilated human neonates. Pediatr Res 2009;66:191–6.
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO . Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177.
Mshvildadze M, Neu J, Mai V . Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutr Rev 2008;66:658–63.
Sharma R, Young C, Neu J . Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol 2010;2010:305879.
Aderem A, Ulevitch RJ . Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–7.
Cho I, Blaser MJ . The human microbiome: at the interface of health and disease. Nat Rev Genet 2012;13:260–70.
Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One 2010;5:e8578.
Jumpstart Consortium Human Microbiome Project Data Generation Working Group. Evaluation of 16S rDNA-based community profiling for human microbiome research. PLoS One 2012;7:e39315.
Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6.
Riehle K, Coarfa C, Jackson A, et al. The Genboree Microbiome Toolset and the analysis of 16S rRNA microbial sequences. BMC Bioinformatics 2012;13:Suppl 13:S11.
Li W, Godzik A . Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006;22:1658–9.
Shannon CE, Weaver W . The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press, 1949.
Acknowledgements
The authors thank Paula Revell, the Texas Children’s Microbiome Center, and the Microbiology Laboratory, especially Jessica K. Runge and Megan Amerson for their invaluable help with specimen processing, and Michelle Rubio-Gonzalez for database management. This work would not have been made possible without the assistance of bedside nurses and respiratory therapists delivering excellent patient care and aiding with specimen collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lohmann, P., Luna, R., Hollister, E. et al. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res 76, 294–301 (2014). https://doi.org/10.1038/pr.2014.85
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2014.85
This article is cited by
-
Lung microbiome: new insights into the pathogenesis of respiratory diseases
Signal Transduction and Targeted Therapy (2024)
-
Perinatal origins of bronchopulmonary dysplasia—deciphering normal and impaired lung development cell by cell
Molecular and Cellular Pediatrics (2023)
-
Hyperoxia exposure upregulates Dvl-1 and activates Wnt/β-catenin signaling pathway in newborn rat lung
BMC Molecular and Cell Biology (2023)
-
Longitudinal development of the airway metagenome of preterm very low birth weight infants during the first two years of life
ISME Communications (2023)
-
Effect of invasive mechanical ventilation on the diversity of the pulmonary microbiota
Critical Care (2022)