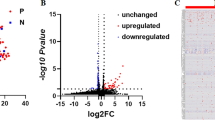
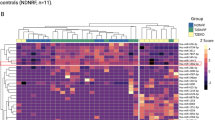
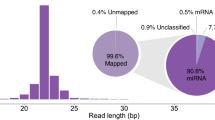
Abstract
Background:
Congenital obstructive nephropathy (CON) is a leading cause of pediatric chronic kidney disease (CKD). Despite optimal surgical and medical care, there is a high rate of CKD progression. Better understanding of molecular and cellular changes is needed to facilitate development of improved biomarkers and novel therapeutic approaches in CON.
Methods:
The megabladder (mgb) mouse is an animal model of CKD with impaired bladder emptying, hydronephrosis, and progressive renal injury. In this study, we characterize a particular microRNA, miR-205, whose expression changes with the degree of hydronephrosis in the mgb−/− kidney.
Results:
Expression of miR-205 is progressively increased in the adult mgb−/− mouse with worsening severity of hydronephrosis. miR-205 expression is correlated with altered expression of cytokeratins and uroplakins, which are markers of cellular differentiation in urothelium. We describe the spatial pattern of miR-205 expression, including increased expression in renal urothelium and novel miR-205 expression in medullary collecting duct epithelium in the congenitally obstructed kidney.
Conclusion:
miR-205 is increased with severity of CON and CKD in the mgb−/− mouse and may regulate urothelial differentiation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Collins AJ, Foley R, Herzog C, et al. Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis 2008;51(1 Suppl 1):S1–320.
Ingraham SE, McHugh KM. Current perspectives on congenital obstructive nephropathy. Pediatr Nephrol 2011;26:1453–61.
Miklovicova D, Cervenova O, Cernianska A, Jancovicova Z, Dedik L, Vasilenkova A. Long-term follow-up of renal function in patients after surgery for obstructive uropathy. Pediatr Nephrol 2008;23:937–45.
Craven AM, Hawley CM, McDonald SP, Rosman JB, Brown FG, Johnson DW. Predictors of renal recovery in Australian and New Zealand end-stage renal failure patients treated with peritoneal dialysis. Perit Dial Int 2007;27:184–91.
Ingraham SE, Saha M, Carpenter AR, et al. Pathogenesis of renal injury in the megabladder mouse: a genetic model of congenital obstructive nephropathy. Pediatr Res 2010;68:500–7.
Singh S, Robinson M, Nahi F, et al. Identification of a unique transgenic mouse line that develops megabladder, obstructive uropathy, and renal dysfunction. J Am Soc Nephrol 2007;18:461–71.
Becknell B, Carpenter AR, Allen JL, et al. Molecular basis of renal adaptation in a murine model of congenital obstructive nephropathy. PLoS One 2013;8:e72762.
McHugh KM. Megabladder mouse model of congenital obstructive nephropathy: genetic etiology and renal adaptation. Pediatr Nephrol 2014;29:645–50.
Akkina S, Becker BN. MicroRNAs in kidney function and disease. Transl Res 2011;157:236–40.
Chandrasekaran K, Karolina DS, Sepramaniam S, et al. Role of microRNAs in kidney homeostasis and disease. Kidney Int 2012;81:617–27.
Li JY, Yong TY, Michael MZ, Gleadle JM. Review: The role of microRNAs in kidney disease. Nephrology (Carlton) 2010;15:599–608.
Saal S, Harvey SJ. MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens 2009;18:317–23.
Orang AV, Safaralizadeh R, Hosseinpour Feizi MA. Insights into the diverse roles of miR-205 in human cancers. Asian Pac J Cancer Prev 2014;15:577–83.
Paterson EL, Kolesnikoff N, Gregory PA, Bert AG, Khew-Goodall Y, Goodall GJ. The microRNA-200 family regulates epithelial to mesenchymal transition. ScientificWorldJournal 2008;8:901–4.
Qin AY, Zhang XW, Liu L, et al. MiR-205 in cancer: an angel or a devil? Eur J Cell Biol 2013;92:54–60.
Vosgha H, Salajegheh A, Smith RA, Lam AK. The important roles of miR-205 in normal physiology, cancers and as a potential therapeutic target. Curr Cancer Drug Targets 2014;14:621–37.
Cai X, Xia Z, Zhang C, et al. Serum microRNAs levels in primary focal segmental glomerulosclerosis. Pediatr Nephrol 2013;28:1797–801.
Wang G, Kwan BC, Lai FM, et al. Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest 2010;90:98–103.
Wang G, Kwan BC, Lai FM, et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens 2010;23:78–84.
Glowacki F, Savary G, Gnemmi V, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 2013;8:e58014.
Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 1988;82:2114–26.
Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frøkiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 1999;10:647–63.
Chung PJ, Chi LM, Chen CL, et al. MicroRNA-205 targets tight junction-related proteins during urothelial cellular differentiation. Mol Cell Proteomics 2014;13:2321–36.
Farmer DT, Shariat N, Park CY, Liu HJ, Mavropoulos A, McManus MT. Partially penetrant postnatal lethality of an epithelial specific microRNA in a mouse knockout. PLoS One 2013;8:e76634.
Wang D, Zhang Z, O’Loughlin E, et al. MicroRNA-205 controls neonatal expansion of skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol 2013;15:1153–63.
Ben-Dov IZ, Tan YC, Morozov P, et al. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS One 2014;9:e86856.
Burbige KA, Lebowitz RL, Colodny AH, Bauer SB, Retik AB. The megacystis-megaureter syndrome. J Urol 1984;131:1133–6.
WILLIAMS DI. Megacystis and megaureter in children. Bull N Y Acad Med 1959;35:317–27.
Kwon TH, Frøkiær J, Nielsen S. Regulation of aquaporin-2 in the kidney: a molecular mechanism of body-water homeostasis. Kidney Res Clin Pract 2013;32:96–102.
Greene SB, Herschkowitz JI, Rosen JM. The ups and downs of miR-205: identifying the roles of miR-205 in mammary gland development and breast cancer. RNA Biol 2010;7:300–4.
Ning MS, Andl T. Concise review: custodians of the transcriptome: how microRNAs guard stemness in squamous epithelia. Stem Cells 2015;33:1047–54.
Kriebel S, Schmidt D, Holdenrieder S, et al. Analysis of tissue and serum microRNA expression in patients with upper urinary tract urothelial cancer. PLoS One 2015;10:e0117284.
Tran MN, Choi W, Wszolek MF, et al. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem 2013;288:3275–88.
Tian C, Liu Q, Ma K, et al. Characterization of induced neural progenitors from skin fibroblasts by a novel combination of defined factors. Sci Rep 2013;3:1345.
Muratsu-Ikeda S, Nangaku M, Ikeda Y, Tanaka T, Wada T, Inagi R. Downregulation of miR-205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLoS One 2012;7:e41462.
Gandellini P, Profumo V, Casamichele A, et al. miR-205 regulates basement membrane deposition in human prostate: implications for cancer development. Cell Death Differ 2012;19:1750–60.
Butt MJ, Tarantal AF, Jimenez DF, Matsell DG. Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int 2007;72:936–44.
Brown D. Tight junctions: guardians of the paracellular pathway. Kidney Int 2000;57:2652–3.
Hemmi A, Mori Y. Immunohistochemical study of cytokeratin distribution in the collecting duct of the human kidney. Acta Pathol Jpn 1991;41:516–20.
Carpenter AR, Becknell B, Ingraham SE, McHugh KM. Ultrasound imaging of the murine kidney. Methods Mol Biol 2012;886:403–10.
Acknowledgements
The authors gratefully acknowledge the assistance of Jamie Mollett in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilhide, M., Feller, J., Li, B. et al. Renal epithelial miR-205 expression correlates with disease severity in a mouse model of congenital obstructive nephropathy. Pediatr Res 80, 602–609 (2016). https://doi.org/10.1038/pr.2016.121
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.121
This article is cited by
-
Epigenetics of kidney disease
Cell and Tissue Research (2017)