Abstract
Background:
Oral propranolol reduces retinopathy of prematurity (ROP) progression, although not safely. This study evaluated safety and efficacy of propranolol eye micro-drops in preterm newborns with ROP.
Methods:
A multicenter open-label trial, planned according to the Simon optimal two-stage design, was performed to analyze safety and efficacy of propranolol micro-drops in newborns with stage 2 ROP. To this end, hemodynamic and respiratory parameters were monitored, and blood samples were collected weekly, for 3 wk. Propranolol plasma levels were also monitored. The progression of the disease was evaluated with serial ophthalmologic examinations.
Results:
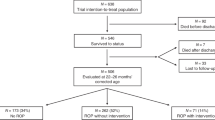
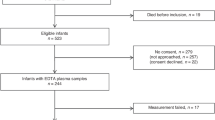
Twenty-three newborns were enrolled. Since the fourth of the first 19 newborns enrolled in the first stage of the study showed a progression to stage 2 or 3 with plus, the second stage was prematurely discontinued. Even though the objective to complete the second stage was not achieved, the percentage of ROP progression (26%) was similar to that obtained previously with oral propranolol administration. However, no adverse effects were observed and propranolol plasma levels were significantly lower than those measured after oral administration.
Conclusion:
Propranolol 0.1% eye micro-drops are well tolerated, but not sufficiently effective. Further studies are required to identify the optimal dose and administration schedule.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74 Suppl 1:35–49.
Gilbert CE, Canovas R, Kocksch de Canovas R, Foster A. Causes of blindness and severe visual impairment in children in Chile. Dev Med Child Neurol 1994;36:326–33.
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008;84:77–82.
Gilbert C, Fielder A, Gordillo L, et al.; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;115:e518–25.
Zepeda-Romero LC, Barrera-de-Leon JC, Camacho-Choza C, et al. Retinopathy of prematurity as a major cause of severe visual impairment and blindness in children in schools for the blind in Guadalajara city, Mexico. Br J Ophthalmol 2011;95:1502–5.
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96.
Scott A, Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond) 2010;24:416–21.
Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–11.
Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10:133–40.
Ristori C, Filippi L, Dal Monte M, et al. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest Ophthalmol Vis Sci 2011;52:155–70.
Dal Monte M, Martini D, Latina V, Pavan B, Filippi L, Bagnoli P. Beta-adrenoreceptor agonism influences retinal responses to hypoxia in a model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2012;53:2181–92.
Martini D, Monte MD, Ristori C, et al. Antiangiogenic effects of β2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J Neurochem 2011;119:1317–29.
Dal Monte M, Cammalleri M, Mattei E, Filippi L, Bagnoli P. Protective effects of β1/2 adrenergic receptor deletion in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2014;56:59–73.
Chen J, Joyal JS, Hatton CJ, et al. Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularization in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2012;53:2968–77.
Chan CK, Pham LN, Zhou J, Spee C, Ryan SJ, Hinton DR. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab Invest 2005;85:721–33.
Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes–characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 2004;369:151–9.
Filippi L, Dal Monte M, Bagnoli P. Different efficacy of propranolol in mice with oxygen-induced retinopathy: could differential effects of propranolol be related to differences in mouse strains? Invest Ophthalmol Vis Sci 2012;53:7421–3.
Wong KK, Potts JE, Etheridge SP, Sanatani S. Medications used to manage supraventricular tachycardia in the infant a North American survey. Pediatr Cardiol 2006;27:199–203.
Smith C, Thomsett M, Choong C, Rodda C, McIntyre HD, Cotterill AM. Congenital thyrotoxicosis in premature infants. Clin Endocrinol (Oxf) 2001;54:371–6.
Garin EH, Araya CE. Treatment of systemic hypertension in children and adolescents. Curr Opin Pediatr 2009;21:600–4.
Mahmoud AB, Tantawy AE, Kouatli AA, Baslaim GM. Propranolol: a new indication for an old drug in preventing postoperative junctional ectopic tachycardia after surgical repair of tetralogy of Fallot. Interact Cardiovasc Thorac Surg 2008;7:184–7.
Filippi L, Cavallaro G, Bagnoli P, et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr 2013;163:1570–1577.e6.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of the Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123:991–9.
Makhoul IR, Peleg O, Miller B, et al. Oral propranolol vs placebo for retinopathy of prematurity: a pilot, randomised, double-blind prospective study. Arch Dis Child 2013;98:565–7.
Bancalari A, Schade R, Muñoz T, Lazcano C, Parada R, Peña R . Oral propranolol in early stages of retinopathy of prematurity. J Perinat Med 2016 Jul 1;44(5):499–503.
Korkmaz L, Baştuğ O, Ozdemir A, Korkut S, Karaca C, Akin MA, Gunes T, Kurtoglu S, Ozturk MA . The Efficacy of Propranolol in Retinopathy of Prematurity and its Correlation with the Platelet Mass Index. Curr Eye Res. 2016 Jun 3:1–10.
Dal Monte M, Casini G, la Marca G, Isacchi B, Filippi L, Bagnoli P. Eye drop propranolol administration promotes the recovery of oxygen-induced retinopathy in mice. Exp Eye Res 2013;111:27–35.
Padrini L, Isacchi B, Bilia AR, et al. Pharmacokinetics and local safety profile of propranolol eye drops in rabbits. Pediatr Res 2014;76:378–85.
Fierson WM ; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013;131:189–95.
Good WV ; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233–48; discussion 248–50.
Nuntnarumit P, Yang W, Bada-Ellzey HS. Blood pressure measurements in the newborn. Clin Perinatol 1999;26:981–96, x.
Della Bona ML, Malvagia S, Villanelli F, et al. A rapid liquid chromatography tandem mass spectrometry-based method for measuring propranolol on dried blood spots. J Pharm Biomed Anal 2013;78-79:34–8.
Filippi L, Cavallaro G, Fiorini P, et al. Propranolol concentrations after oral administration in term and preterm neonates. J Matern Fetal Neonatal Med 2013;26:833–40.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10.
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51.
Praveen V, Vidavalur R, Rosenkrantz TS, Hussain N. Infantile hemangiomas and retinopathy of prematurity: possible association. Pediatrics 2009;123:e484–9.
Filippi L, Dal Monte M, Casini G, Daniotti M, Sereni F, Bagnoli P. Infantile hemangiomas, retinopathy of prematurity and cancer: a common pathogenetic role of the β-adrenergic system. Med Res Rev 2015;35:619–52.
File RR, Patton TF. Topically applied pilocarpine. Human pupillary response as a function of drop size. Arch Ophthalmol 1980;98:112–5.
Elibol O, Alçelik T, Yüksel N, Caglar Y. The influence of drop size of cyclopentolate, phenylephrine and tropicamide on pupil dilatation and systemic side effects in infants. Acta Ophthalmol Scand 1997;75:178–80.
Kumar S, Karki R, Meena M, Prakash T, Rajeswari T, Goli D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Technol Res 2011;2:192–4.
Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol 1982;26:207–18.
Dal Monte M, Filippi L, Bagnoli P. Beta3-adrenergic receptors modulate vascular endothelial growth factor release in response to hypoxia through the nitric oxide pathway in mouse retinal explants. Naunyn Schmiedebergs Arch Pharmacol 2013;386:269–78.
Good WV, Hardy RJ, Wallace DK, et al. β-Blocking and racial variation in the severity of retinopathy of prematurity. Arch Ophthalmol 2012;130:117–8.
Acknowledgements
We are most grateful to the nursing staff of the Neonatal Intensive Care Units of the A. Meyer University Children’s Hospital, Florence, the University Hospital of Siena, and the Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, University of Milan, Italy, for their assistance in conducting this study. Trial Registration: Current Controlled Trials ISRCTN68126628; ClinicalTrials.gov Identifier NCT02014454; EudraCT Number 2013-002062-39.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures S1
(TIFF 2015 kb)
Supplementary Figures S2
(TIFF 1675 kb)
Supplementary Table S1
(DOC 52 kb)
Rights and permissions
About this article
Cite this article
Filippi, L., Cavallaro, G., Bagnoli, P. et al. Propranolol 0.1% eye micro-drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr Res 81, 307–314 (2017). https://doi.org/10.1038/pr.2016.230
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.230
This article is cited by
-
Optimizing instilled drug delivery: a scoping review of microdrops in ophthalmology
Graefe's Archive for Clinical and Experimental Ophthalmology (2025)
-
Comparison of Different Doses of Oral and Ocular Propranolol for Retinopathy of Prematurity: A Network Meta-Analysis
Pediatric Drugs (2024)
-
The efficacy and safety of inositol supplementation in preterm infants to prevent retinopathy of prematurity: a systematic review and meta-analysis
BMC Ophthalmology (2019)
-
Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis
Journal of Perinatology (2019)
-
Study protocol: safety and efficacy of propranolol 0.2% eye drops in newborns with a precocious stage of retinopathy of prematurity (DROP-ROP-0.2%): a multicenter, open-label, single arm, phase II trial
BMC Pediatrics (2017)