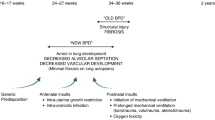
Abstract
Lung diseases remain one of the main causes of morbidity and mortality in neonates. Cell therapy and regenerative medicine have the potential to revolutionize the management of life-threatening and debilitating lung diseases that currently lack effective treatments. Over the past decade, the repair capabilities of stem/progenitor cells have been harnessed to prevent/rescue lung damage in experimental neonatal lung diseases. Mesenchymal stromal cells and amnion epithelial cells exert pleiotropic effects and represent ideal therapeutic cells for bronchopulmonary dysplasia, a multifactorial disease. Endothelial progenitor cells are optimally suited to promote lung vascular growth and attenuate pulmonary hypertension in infants with congenital diaphragmatic hernia or a vascular bronchopulmonary dysplasia phenotype. Induced pluripotent stem cells (iPSCs) are one of the most exciting breakthroughs of the past decade. Patient-specific iPSCs can be derived from somatic cells and differentiated into any cell type. iPSCs can be capitalized upon to develop personalized regenerative cell products for surfactant protein deficiencies—lethal lung disorders without treatment—that affect a single gene in a single cell type and thus lend themselves to phenotype-specific cell replacement. While the clinical translation has begun, more needs to be learned about the biology of these repair cells to make this translation successful.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Owen LS, Manley BJ, Davis PG, Doyle LW . The evolution of modern respiratory care for preterm infants. Lancet 2017;389:1649–1659.
Stoll BJ, Hansen NI, Bell EF et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–1051.
Abman SH . Bronchopulmonary dysplasia: "a vascular hypothesis”. Am J Respir Crit Care Med 2001;164:1755–1756.
Khemani E, McElhinney DB, Rhein L et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–1269.
Kinsella JP, Ivy DD, Abman SH . Pulmonary vasodilator therapy in congenital diaphragmatic hernia: acute, late, and chronic pulmonary hypertension. Semin Perinatol 2005;29:123–128.
Lusk LA, Wai KC, Moon-Grady AJ, Steurer MA, Keller RL . Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. J Pediatr 2015;166:251–256 and 251.
Nogee LM . Interstitial lung disease in newborns. Semin Fetal Neonatal Med 2017;22:227–233.
McCulloch EA, Till JE . The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res 1960;13:115–125.
Atkins H, Freedman MS . Immunoablation and aHSCT for aggressive multiple sclerosis—Authors' reply. Lancet 2017;389:908.
le Cras TD, Markham NE, Morris KG, Ahrens CR, McMurtry IF, Abman SH . Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am J Physiol Lung Cell Mol Physiol 2000;278:L822–829.
Friedenstein AJ, Gorskaja JF, Kulagina NN . Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267–274.
Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317.
Wagner DE, Cardoso WV, Gilpin SE et al. An Official American Thoracic Society Workshop Report 2015. Stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc 2016;13:S259–S278.
Aslam M, Baveja R, Liang OD et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 2009;180:1122–1130.
van Haaften T, Byrne R, Bonnet S et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131–1142.
Hsieh JY, Wang HW, Chang SJ et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 2013;8:e72604.
Jin HJ, Bae YK, Kim M et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Intern J Mol Sci 2013;14:17986–18001.
Yannarelli G, Dayan V, Pacienza N, Lee CJ, Medin J, Keating A . Human umbilical cord perivascular cells exhibit enhanced cardiomyocyte reprogramming and cardiac function after experimental acute myocardial infarction. Cell Transplant 2013;22:1651–1666.
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA . Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764.
Chang YS, Choi SJ, Ahn SY et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS ONE 2013;8:e52419.
Pierro M, Ionescu L, Montemurro T et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013;68 ((5)): 475–484.
Fung ME, Thebaud B . Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatr Res 2014;75:2–7.
Chang YS, Ahn SY, Jeon HB et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol 2014;51:391–399.
Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B . Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev 2012;21:2789–2797.
Kourembanas S . Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 2015;77:13–27.
Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U . Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod 2007;77:577–588.
Vosdoganes P, Hodges RJ, Lim R et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol 2011;205:156 and 126–133.
Vosdoganes P, Lim R, Koulaeva E et al. Human amnion epithelial cells modulate hyperoxia-induced neonatal lung injury in mice. Cytotherapy 2013;15:1021–1029.
Vosdoganes P, Wallace EM, Chan ST, Acharya R, Moss TJ, Lim R . Human amnion epithelial cells repair established lung injury. Cell Transplant 2013;22:1337–1349.
Chang YS, Ahn SY, Yoo HS et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 2014;164:966–972 and 966.
Ahn SY, Chang YS, Kim JH, Sung SI, Park WS . Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr 2017;185:49–54.
Boregowda SV, Phinney DG . Quantifiable metrics for predicting MSC therapeutic efficacy. J Stem Cell Res Ther 2016;6:6. pii: 365.
Martin I, De Boer J, Sensebe L, MSC Committee of the International Society for Cellular Therapy. A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy 2016;18:613–620.
Thebaud B, Abman SH . Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175:978–985.
Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967.
Medina RJ, Barber CL, Sabatier F et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 2017;6:1316–1320.
Ingram DA, Mead LE, Tanaka H et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760.
Alvarez DF, Huang L, King JA, Elzarrad MK, Yoder MC, Stevens T . Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 2008;294:L419–430.
Alphonse RS, Vadivel A, Zhong S et al. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nature Protoc 2015;10:1697–1708.
Solomon I, O'Reilly M, Ionescu L et al. Functional differences between placental micro- and macrovascular endothelial colony-forming cells. Stem Cells Transl Med 2016;5:291–300.
Alphonse RS, Vadivel A, Fung M et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 2014;129:2144–2157.
Baker CD, Balasubramaniam V, Mourani PM et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J 2012;40:1516–1522.
Borghesi A, Massa M, Campanelli R et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2009;180:540–546.
Baker CD, Ryan SL, Ingram DA et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med 2009;180:454–461.
Baker CD, Black CP, Ryan SL, Balasubramaniam V, Abman SH . Cord blood endothelial colony-forming cells from newborns with congenital diaphragmatic hernia. J Pediatr 2013;163:905–907.
Fujinaga H, Fujinaga H, Watanabe N et al. Cord blood-derived endothelial colony-forming cell function is disrupted in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 2016;310:L1143–1154.
Bertagnolli M, Nuyt AM, Thebaud B, Luu TM . Endothelial progenitor cells as prognostic markers of preterm birth-associated complications. Stem Cells Transl Med 2017;6 ((1)): 7–13.
Baker CD, Seedorf GJ, Wisniewski BL et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2013;305:L73–81.
Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH . Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292:L1073–1084.
Balasubramaniam V, Ryan SL, Seedorf GJ et al. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 2010;298:L315–323.
Gurdon JB . The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol 1962;10:622–640.
Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676.
Roszell B, Mondrinos MJ, Seaton A et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A 2009;15:3351–3365.
Hawkins F, Kramer P, Jacob A et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J Clin Invest 2017;127:2277–2294.
Longmire TA, Ikonomou L, Hawkins F et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 2012;10:398–411.
Mou H, Zhao R, Sherwood R et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 2012;10:385–397.
Ghaedi M, Calle EA, Mendez JJ et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 2013;123:4950–4962.
Huang SX, Islam MN, O'Neill J et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 2014;32:84–91.
Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL . Lung organoids: current uses and future promise. Development 2017;144:986–997.
Chen YW, Huang SX, de Carvalho A et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 2017;19:542–549.
Wilkinson DC, Alva-Ornelas JA, Sucre JM et al. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 2017;6:622–633.
Crane AM, Kramer P, Bui JH et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep 2015;4:569–577.
Firth AL, Menon T, Parker GS et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 2015;12:1385–1390.
McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN . Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 2017;20:844–857 e846.
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER . Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;126:332–337.
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER . Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;125:740–745.
Melton KR, Nesslein LL, Ikegami M et al. SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol 2003;285:L543–549.
Soh BS, Zheng D, Li Yeo JS et al. CD166pos subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Mol Ther 2012;20:2335–2346.
Mahiny AJ, Dewerth A, Mays LE et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol 2015;33:584–586.
Mandai M, Watanabe A, Kurimoto Y et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–1046.
Kilpinen H, Goncalves A, Leha A et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 2017;546:370–375.
Merkle FT, Ghosh S, Kamitaki N et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 2017;545:229–233.
Beers MF, Moodley Y . When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. Am J Respir Cell Mol Biol 2017;57:18–27.
Zhao T, Zhang ZN, Rong Z, Xu Y . Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–215.
Daley GQ . Polar extremes in the clinical use of stem cells. N Engl J Med 2017;376:1075–1077.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support and Disclosure
B.T. is supported by the Canadian Institute of Health Research, Canadian Thoracic Society, Ontario Institute for Regenerative Medicine, and the Children’s Hospital of Eastern Ontario foundation.
Rights and permissions
About this article
Cite this article
Kang, M., Thébaud, B. Stem cell biology and regenerative medicine for neonatal lung diseases. Pediatr Res 83, 291–297 (2018). https://doi.org/10.1038/pr.2017.232
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.232
This article is cited by
-
Human amniotic epithelial stem cells, a potential therapeutic approach for diabetes and its related complications
Human Cell (2025)
-
Fetal endothelial colony-forming cell impairment after maternal kidney transplantation
Pediatric Research (2023)
-
Emerging antenatal therapies for congenital diaphragmatic hernia-induced pulmonary hypertension in preclinical models
Pediatric Research (2021)
-
STAT3–BDNF–TrkB signalling promotes alveolar epithelial regeneration after lung injury
Nature Cell Biology (2020)
-
Preventing bronchopulmonary dysplasia: new tools for an old challenge
Pediatric Research (2019)