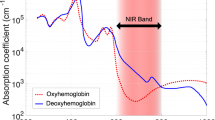
Abstract
Background
Development of cerebral edema after brain injury carries a high risk for brain damage and death. The present study tests the ability of a noninvasive cerebral edema monitoring system that uses near-infrared spectroscopy (NIRS) with water as the chromophore of interest to detect brain edema following hypoxia.
Methods
Ventilated piglets were exposed to hypoxia for 1 h, and then returned to normal oxygen levels for 4 h. An NIRS sensor was placed on the animal’s head at baseline, and changes in light attenuation were converted to changes in H2O. Cerebral water content and aquaporin-4 protein (AQP4) expression were measured.
Results
The system detected changes in NIRS–derived water signal as early as 2 h after hypoxia, and provided fivefold signal amplification, representing a 10% increase in brain water content and a sixfold increase in AQP4, 4 h after hypoxia. Changes in water signal correlated well with changes in cerebral water content (R=0.74) and AQP4 expression (R=0.97) in the piglet brain.
Conclusion
The data show that NIRS can detect cerebral edema early in the injury process, thus providing an opportunity to initiate therapy at an earlier and more effective time-point after an insult than is available with current technology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kurinczuk JJ, White-Koning M, Badawi N . Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum Dev 2010;86:329–338.
Hinduja A, Gupta H, Yang JD et al. Hypoxic ischemic brain injury following in hospital cardiac arrest—lessons from autopsy. J Forensic Leg Med 2014;23:84–86.
Eunson P . The long-term health, social, and financial burden of hypoxic–ischaemic encephalopathy. Dev Med Child Neurol 2015;57 (Suppl 3): 48–50.
Nag S, Manias JL, Stewart DJ . Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol 2009;118:197–217.
Pasantes-Morales H, Vazquez-Juarez E . Transporters and channels in cytotoxic astrocyte swelling. Neurochem Res 2012;37:2379–2387.
Benga G . Water channel proteins: from their discovery in 1985 in Cluj-Napoca, Romania, to the 2003 Nobel Prize in Chemistry. Cell Mol Biol (Noisy-le-grand) 2006;52:10–19.
Amiry-Moghaddam M, Frydenlund DS, Ottersen OP . Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004;129:999–1010.
Potokar M, Jorgacevski J, Zorec R . Astrocyte aquaporin dynamics in health and disease. Int J Mol Sci 2016: 17.
Assentoft M, Larsen BR, MacAulay N . Regulation and function of AQP4 in the central nervous system. Neurochem Res 2015;40:2615–2627.
Yukutake Y, Yasui M . Regulation of water permeability through aquaporin-4. Neuroscience 2010;168:885–891.
Feickert HJ, Drommer S, Heyer R . Severe head injury in children: impact of risk factors on outcome. J Trauma 1999;47:33–38.
Lupton BA, Hill A, Roland EH et al. Brain swelling in the asphyxiated term newborn: pathogenesis and outcome. Pediatrics 1988;82:139–146.
Nakagawa TA, Ashwal S, Mathur M et al. Clinical report—Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Pediatrics 2011;128 e720-40.
Becker DP, Miller JD, Ward JD et al. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg 1977;47:491–502.
Bullock MR, Povlishock JT . Guidelines for the management of severe traumatic brain injury. Editor’s Commentary. J Neurotrauma 2007;24 (Suppl 1): 2 p preceding S1.
Cope M, Delpy DT . System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput 1988;26:289–294.
Ashraf QM, Zubrow AB, Mishra OP et al. Nitration of Bax and Bcl-2 proteins during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibition. Biol Neonate 2002;81:65–72.
Izzetoglu M, Bunce SC, Izzetoglu K et al. Functional brain imaging using near-infrared technology. IEEE Eng Med Biol Mag 2007;26:38–46.
Bozkurt A, Rosen A, Rosen H et al. A portable near infrared spectroscopy system for bedside monitoring of newborn brain. Biomed Eng Online 2005;4:29.
Wyatt JS, Cope M, Delpy DT et al. Measurement of optical path length for cerebral near-infrared spectroscopy in newborn infants. Dev Neurosci 1990;12:140–144.
DiGiacomo JE, Pane CR, Gwiazdowski S et al. Effect of graded hypoxia on brain cell membrane injury in newborn piglets. Biol Neonate 1992;61:25–32.
Thoresen M, Haaland K, Loberg EM et al. A piglet survival model of posthypoxic encephalopathy. Pediatr Res 1996;40:738–748.
Ashraf QM, Haider SH, Katsetos CD et al. Nitric oxide-mediated alterations of protein tyrosine phosphatase activity and expression during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett 2004;362:108–112.
Mami AG, Ballesteros JR, Fritz KI et al. Effects of magnesium sulfate administration during hypoxia on CaM kinase IV and protein tyrosine kinase activities in the cerebral cortex of newborn piglets. Neurochem Res 2006;31:57–62.
Mishra OP, Delivoria-Papadopoulos M . Effect of hypoxia on protein tyrosine kinase activity in cortical membranes of newborn piglets—the role of nitric oxide. Neurosci Lett 2004;372:114–118.
Lingwood BE, Dunster KR, Colditz PB et al. Noninvasive measurement of cerebral bioimpedance for detection of cerebral edema in the neonatal piglet. Brain Res 2002;945:97–105.
Thiagarajah JR, Papadopoulos MC, Verkman AS . Noninvasive early detection of brain edema in mice by near-infrared light scattering. J Neurosci Res 2005;80:293–299.
Qiao M, Meng S, Scobie K et al. Magnetic resonance imaging of differential gray versus white matter injury following a mild or moderate hypoxic-ischemic insult in neonatal rats. Neurosci Lett 2004;368:332–336.
Thornton JS, Ordidge RJ, Penrice J et al. Temporal and anatomical variations of brain water apparent diffusion coefficient in perinatal cerebral hypoxic–ischemic injury: relationships to cerebral energy metabolism. Magn Reson Med 1998;39:920–927.
Kim JJ, Gean AD . Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics 2011;8:39–53.
Lee B, Newberg A . Neuroimaging in traumatic brain imaging. NeuroRx 2005;2:372–383.
Kudreviciene A, Basevicius A, Lukosevicius S et al. The value of ultrasonography and Doppler sonography in prognosticating long-term outcomes among full-term newborns with perinatal asphyxia. Medicina (Kaunas) 2014;50:100–110.
Pinto PS, Tekes A, Singhi S et al. White-gray matter echogenicity ratio and resistive index: sonographic bedside markers of cerebral hypoxic-ischemic injury/edema? J Perinatol 2012;32:448–453.
Hanlo PW, Gooskens RH, Nijhuis IJ et al. Value of transcranial Doppler indices in predicting raised ICP in infantile hydrocephalus. A study with review of the literature. Childs Nerv Syst 1995;11:595–603.
Tzeng YC, Ainslie PN, Cooke WH et al. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol 2012;303:H658–H671.
Acknowledgements
We thank Baruch Ben Dor, John Grothusen, Juan Du, and Jessica Button for their technical assistance. This project was funded, in part, under a grant with the Pennsylvania Department of Health SAP Number: 4100068711, and by the Coulter-Drexel Translational Research Partnership Program under grant number: 282812-3850.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of financial support
This project was funded, in part, under a grant with the Pennsylvania Department of Health SAP Number: 4100068711, and by the Coulter-Drexel Translational Research Partnership Program under grant number: 282812-3850.
Rights and permissions
About this article
Cite this article
Malaeb, S., Izzetoglu, M., McGowan, J. et al. Noninvasive monitoring of brain edema after hypoxia in newborn piglets. Pediatr Res 83, 484–490 (2018). https://doi.org/10.1038/pr.2017.264
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.264