Abstract
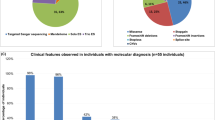
Many female carriers of Fabry disease are likely to develop severe morbidity and mortality. However, by our own estimation, around 80% of female newborns are missed by our current enzyme-based screening approach. Our team’s aim was to develop an improved cost-effective screening method that is able to detect Fabry disease among female newborns. In Taiwan, based on a database of 916,000 newborns, ~98% of Fabry patients carry mutations out of a pool of only 21 pathogenic mutations. An Agena iPLEX platform was designed to detect these 21 pathogenic mutations using only a single-assay panel. A total of 54,791 female infants were screened and 136 female newborns with the IVS4 + 919G > A mutation and one female newborn with the c.656T > C mutation were identified. Using the current enzyme-based newborn screening approach as baseline, around 83% of female newborns are being missed. Through a family study of the IVS4 female newborns, 30 IVS4 adult family members were found to have left ventricular hypertrophy. Ten patients received endomyocardial biopsy and all were found to have significant globotriaosylceramide (Gb3) accumulation in their cardiomyocytes. All of these individuals now receive enzyme replacement therapy. We have demonstrated that the Agena iPLEX assay is a powerful tool for detecting females with Fabry disease. Furthermore, through this screening, we also have been able to identify many disease-onset adult family members who were originally undiagnosed for Fabry disease. This screening helps them to receive treatment in time before severe and irreversible cardiac damage has occurred.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Desnick RJ, Ioannou YA, Eng CM a-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York, USA: McGraw-Hill, 2001. p. 3733–74
Clarke JT. Narrative review: Fabry disease. Ann Intern Med. 2007;146:425–33.
Desnick RJ, Wasserstein MP. Fabry disease: clinical features and recent advances in enzyme replacement therapy. Adv Nephrol Necker Hosp. 2001;31:317–39.
Desnick RJ, Brady RO. Fabry disease in childhood. J Pediatr. 2004;144:S20–6.
Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372:1427–35.
Weidemann F, Linhart A, Monserrat L, Strotmann J. Cardiac challenges in patients with Fabry disease. Int J Cardiol. 2010;141:3–10.
Bhatia GS, Leahy JF, Connolly DL, Davis RC. Severe left ventricular hypertrophy in Anderson-Fabry disease. Heart. 2004;90:1136.
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–93.
Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–11.
Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64:801–7.
Rolfs A, Bottcher T, Zshiesche M, Morris P, Winchester B, Bauer P, et al. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–6.
Nance CS, Klein CJ, Banikazemi M, Dikman SH, Phelps RG, McArthur JC, et al. Later-onset Fabry disease: an adult variant presenting with the cramp-fasciculation syndrome. Arch Neurol. 2006;63:453–7.
Nagueh SF. Fabry disease. Heart. 2003;89:819–20.
Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–6.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54.
Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503.
Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S. Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet. 2013;58:548–52.
Wittmann J, Karg E, Turi S, Legnini E, Wittmann G, Giese AK, et al. Newborn screening for lysosomal storage disorders in hungary. JIMD Rep. 2012;6:117–25.
Mechtler TP, Metz TF, Muller HG, Ostermann K, Ratschmann R, De Jesus VR, et al. Short-incubation mass spectrometry assay for lysosomal storage disorders in newborn and high-risk population screening. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;908:9–17.
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40.
Hopkins PV, Campbell C, Klug T, Rogers S, Raburn-Miller J, Kiesling J. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J Pediatr. 2015;166:172–7.
Hsu TR, Hung SC, Chang FP, Yu WC, Sung SH, Hsu CL, et al. Later-onset fabry disease - cardiac damage progresses in Silence - experience with a highly prevalent mutation. J Am Coll Cardiol. 2016;68:2554–63.
Tai CL, Liu MY, Yu HC, Chiang CC, Chiang H, Suen JH, et al. The use of high resolution melting analysis to detect Fabry mutations in heterozygous females via dry bloodspots. Clin Chim Acta. 2012;413:422–7.
Linthorst GE, Vedder AC, Aerts JM, Hollak CE. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin Chim Acta. 2005;353:201–3.
Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51:2082.
Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, et al. Females with Fabry disease frequently have major organ involvement: lessons from the fabry registry. Mol Genet Metab. 2008;93:112–28.
Deegan P, Baehner AF, Barba-Romero MA, Hughes DA & Beck M Fabry disease in females: clinical characteristics and the effects of enzyme replacement therapy. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry disease: perspectives from 5 years of FOS. Oxford, UK: Oxford PharmaGenesis, 2006. p. 295–304.
Gupta S, Ries M, Kotsopoulos S, Schiffmann R. The relationship of vascular glycolipid storage to clinical manifestations of Fabry disease: a cross-sectional study of a large cohort of clinically affected heterozygous women. Medicine. 2005;84:261–8.
Lee SH, Li CF, Lin HY, Lin CH, Liu HC, Tsai SF, et al. High-throughput detection of common sequence variations of Fabry disease in Taiwan using DNA mass spectrometry. Mol Genet Metab. 2014;111:507–12.
Calvo SE, Tucker EJ, Compton AG, Kirby DM, Crawford G, et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat Genet. 2010;42:851–8.
Gabriel S, Ziaugra L, Tabbaa D SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet 2009; Chapter 2: Unit 2.12.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60.
Deegan PB, Baehner AF, Barba-Romero MA, Hughes DA, Kampmann C, Beck M, et al. Natural history of Fabry disease in females in the Fabry outcome survey. J Med Genet. 2006;43:347–52.
Acknowledgements
This work was partially supported by the National Science Council, Taiwan (No. NSC-100-2325-B-010–4) and Taipei Veterans General Hospital (No. V101C-129 and V101C-187).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lu, YH., Huang, PH., Wang, LY. et al. Improvement in the sensitivity of newborn screening for Fabry disease among females through the use of a high-throughput and cost-effective method, DNA mass spectrometry. J Hum Genet 63, 1–8 (2018). https://doi.org/10.1038/s10038-017-0366-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-017-0366-y
This article is cited by
-
Correlation of enzyme activities and genotype with clinical manifestations in Chinese patients of different sexes with classical and late-onset Fabry disease
Frontiers of Medicine (2025)
-
How relevant are cerebral white matter lesions in the D313Y variant of the α-galactosidase A gene? Neurological, cardiological, laboratory, and MRI data of 21 patients within a follow-up of 3 years
Neurological Sciences (2023)
-
Morbus Fabry in der Neurologie
DGNeurologie (2021)
-
Fabry disease screening in high-risk populations in Japan: a nationwide study
Orphanet Journal of Rare Diseases (2020)
-
Prevalence of Fabry disease in dialysis patients: Western Australia Fabry disease screening study - the FoRWARD study
Orphanet Journal of Rare Diseases (2020)