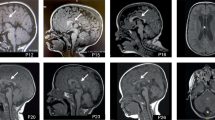
Abstract
Cerebrotendinous xanthomatosis (CTX) is likely to be underdiagnosed and precise epidemiological characteristics of CTX are largely unknown as knowledge on the disorder is based mainly on case reports. We conducted a nationwide survey on CTX to elucidate the frequency, clinical picture, and molecular biological background of Japanese CTX patients. In this first Japanese nationwide survey on CTX, 2541 questionnaires were sent to clinical departments across Japan. A total of 1032 (40.6%) responses were returned completed for further analysis. Forty patients with CTX (50.0% male) were identified between September 2012 and August 2015. The mean age of onset was 24.5 ± 13.6 years, mean age at diagnosis was 41.0 ± 11.6 years, and corresponding mean duration of illness from onset to diagnosis was 16.5 ± 13.5 years. The most common initial symptom was tendon xanthoma, followed next by spastic paraplegia, cognitive dysfunction, cataract, ataxia, and epilepsy. The most predominant mutations in the CYP27A1 gene were c.1214G> A (p.R405Q, 31.6%), c.1421G> A (p.R474Q, 26.3%), and c.435G> T (p.G145=, 15.8%). Therapeutic interventions that included chenodeoxycholic acid, HMG-CoA reductase inhibitor, and LDL apheresis reduced serum cholestanol level in all patients and improved clinical symptoms in 40.5% of patients. Although CTX is a treatable neurodegenerative disorder, our nationwide survey revealed an average 16.5-year diagnostic delay. CTX may be underdiagnosed in Japan, especially during childhood. Early diagnosis and treatment are essential to improve the prognosis of CTX.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991;266:7779–83.
Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266:7774–8.
Verrips A, Hoefsloot LH, Steenbergen GC, Theelen JP, Wevers RA, Gabreels FJ, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123:908–19.
Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143–9.
Gallus GN, Dotti MT, Mignarri A, Rufa A, Da Pozzo P, Cardaioli E, et al. Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur J Neurol. 2010;17:1259–62.
Nozue T, Higashikata T, Inazu A, Kawashiri MA, Nohara A, Kobayashi J, et al. Identification of a novel missense mutation in the sterol 27-hydroxylase gene in two Japanese patients with cerebrotendinous xanthomatosis. Intern Med. 2010;49:1127–31.
Chen W, Kubota S, Kim KS, Cheng J, Kuriyama M, Eggertsen G, et al. Novel homozygous and compound heterozygous mutations of sterol 27-hydroxylase gene (CYP27) cause cerebrotendinous xanthomatosis in three Japanese patients from two unrelated families. J Lipid Res. 1997;38:870–9.
Suh S, Kim HK, Park HD, Ki CS, Kim MY, Jin SM, et al. Three siblings with Cerebrotendinous Xanthomatosis: a novel mutation in the CYP27A1 gene. Eur J Med Genet. 2012;55:71–4.
Lee MH, Hazard S, Carpten JD, Yi S, Cohen J, Gerhardt GT, et al. Fine-mapping, mutation analyses, and structural mapping of cerebrotendinous xanthomatosis in U.S. pedigrees. J Lipid Res. 2001;42:159–69.
Clayton PT, Verrips A, Sistermans E, Mann A, Mieli-Vergani G, Wevers R. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2002;25:501–13.
Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311:1649–52.
Pilo-De-La-Fuente B, Jimenez-Escrig A, Lorenzo JR, Pardo J, Arias M, Ares-Luque A, et al. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol. 2011;18:1203–11.
Mignarri A, Gallus GN, Dotti MT, Federico A. A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2014;37:421–9.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Appadurai V, Debarber A, Chiang PW, Patel SB, Steiner RD, Tyler C, et al. Apparent underdiagnosis of cerebrotendinous xanthomatosis revealed by analysis of ~60,000 human exomes. Mol Genet Metab. 2015;116:298–304.
Van Heijst AF, Verrips A, Wevers RA, Cruysberg JR, Renier WO, Tolboom JJ. Treatment and follow-up of children with cerebrotendinous xanthomatosis. Eur J Pediatr. 1998;157:313–6.
Mondelli M, Sicurelli F, Scarpini C, Dotti MT, Federico A. Cerebrotendinous xanthomatosis: 11-year treatment with chenodeoxycholic acid in five patients. An electrophysiological study. J Neurol Sci. 2001;190:29–33.
Yoshinaga T, Sekijima Y, Koyama S, Maruyama K, Yoshida T, Kato T, et al. Clinical and radiological findings of a cerebrotendinous xanthomatosis patient with a novel p.A335V mutation in the CYP27A1 gene. Intern Med. 2014;53:2725–9.
Peynet J, Laurent A, De Liege P, Lecoz P, Gambert P, Legrand A, et al. Cerebrotendinous xanthomatosis: treatments with simvastatin, lovastatin, and chenodeoxycholic acid in 3 siblings. Neurology. 1991;41:434–6.
Pilo De La Fuente B, Ruiz I, Lopez De Munain A, Jimenez-Escrig A. Cerebrotendinous xanthomatosis: neuropathological findings. J Neurol. 2008;255:839–42.
Pierre G, Setchell K, Blyth J, Preece MA, Chakrapani A, Mckiernan P. Prospective treatment of cerebrotendinous xanthomatosis with cholic acid therapy. J Inherit Metab Dis. 2008;31(Suppl 2):S241–5.
Verrips A, Nijeholt GJ, Barkhof F, Van Engelen BG, Wesseling P, Luyten JA, et al. Spinal xanthomatosis: a variant of cerebrotendinous xanthomatosis. Brain. 1999;122:1589–95.
Abe R, Sekijima Y, Kinoshita T, Yoshinaga T, Koyama S, Kato T, et al. Spinal form cerebrotendinous xanthomatosis patient with long spinal cord lesion. J Spinal Cord Med. 2016;39:726–9.
Yanagihashi M, Kano O, Terashima T, Kawase Y, Hanashiro S, Sawada M, et al. Late-onset spinal form xanthomatosis without brain lesion: a case report. BMC Neurol. 2016;16:21.
Nicholls Z, Hobson E, Martindale J, Shaw PJ. Diagnosis of spinal xanthomatosis by next-generation sequencing: identifying a rare, treatable mimic of hereditary spastic paraparesis. Pract Neurol. 2015;15:280–3.
Saute JA, Giugliani R, Merkens LS, Chiang JP, Debarber AE, De Souza CF. Look carefully to the heels! A potentially treatable cause of spastic paraplegia. J Inherit Metab Dis. 2015;38:363–4.
Schreiner A, Hopen G, Skrede S. Cerebrotendinous xanthomatosis (cholestanolosis). Investigations on two sisters and their family. Acta Neurol Scand. 1975;51:405–16.
Swartz M, Burman KD, Salen G. Cerebrotendinous xanthomatosis: a cause of cataracts and tendon xanthoma. Am J Med Sci. 1982;283:147–52.
Kuriyama M, Fujiyama J, Yoshidome H, Takenaga S, Matsumuro K, Kasama T, et al. Cerebrotendinous xanthomatosis: clinical and biochemical evaluation of eight patients and review of the literature. J Neurol Sci. 1991;102:225–32.
Restuccia D, Di Lazzaro V, Servidei S, Colosimo C, Tonali P. Somatosensory and motor evoked potentials in the assessment of cerebrotendinous xanthomatosis before and after treatment with chenodeoxycholic acid: a preliminary study. J Neurol Sci. 1992;112:139–46.
Dotti MT, Federico A, Signorini E, Caputo N, Venturi C, Filosomi G, et al. Cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease): CT and MR findings. Am J Neuroradiol. 1994;15:1721–6.
Koopman BJ, Wolthers BG, Van Der Molen JC, Waterreus RJ. Bile acid therapies applied to patients suffering from cerebrotendinous xanthomatosis. Clin Chim Acta. 1985;152:115–22.
Kuriyama M, Tokimura Y, Fujiyama J, Utatsu Y, Osame M. Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J Neurol Sci. 1994;125:22–8.
Ito S, Kuwabara S, Sakakibara R, Oki T, Arai H, Oda S, et al. Combined treatment with LDL-apheresis, chenodeoxycholic acid and HMG-CoA reductase inhibitor for cerebrotendinous xanthomatosis. J Neurol Sci. 2003;216:179–82.
Mimura Y, Kuriyama M, Tokimura Y, Fujiyama J, Osame M, Takesako K, et al. Treatment of cerebrotendinous xanthomatosis with low-density lipoprotein (LDL)-apheresis. J Neurol Sci. 1993;114:227–30.
Berginer VM, Salen G. LDL-apheresis cannot be recommended for treatment of cerebrotendinous xanthomatosis. J Neurol Sci. 1994;121:229–32.
Dotti MT, Lutjohann D, Von Bergmann K, Federico A. Normalisation of serum cholestanol concentration in a patient with cerebrotendinous xanthomatosis by combined treatment with chenodeoxycholic acid, simvastatin and LDL apheresis. Neurol Sci. 2004;25:185–91.
Acknowledgements
The authors thank Y. Iwanaka, S. Ito, T. Yoshida, K. Tokuoka, H. Yuasa, M. Takata, S. Hirose, S. Togashi. H. Taniguchi, K. Ishihara, T. Hashimoto, T. Takeshima, S. Akazawa, A. Haraguchi, M. Ito, K. Takase, S. Susa, Y. Honma, H. Kaneko, Y. Touma, K. Yamamoto, Y. Harigaya, K. Tamura, I. Yokota, T. Kawazoe, S. Takagi, T. Hamada, M. Takemoto, H. Nagayama, M. Mizuno, K. Uemura, Y. Kajimoto, and O. Kano for providing clinical patient information. This study was supported by a grant from the Research Committee for Cerebrotendinous Xanthomatosis, Research on Measures against Intractable Diseases by the Japanese Ministry of Health, Labour, and Welfare and a grant from the Research Committee for Primary Hyperlipidemia, Research on Measures against Intractable Diseases by the Japanese Ministry of Health, Labour, and Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sekijima, Y., Koyama, S., Yoshinaga, T. et al. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet 63, 271–280 (2018). https://doi.org/10.1038/s10038-017-0389-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-017-0389-4
This article is cited by
-
Juvenile-Onset Cerebrotendinous Xanthomatosis with Novel Compound Heterozygous CYP27A1 Mutations: Case Series and Literature Review
The Cerebellum (2026)
-
Cholic acid as a treatment for cerebrotendinous xanthomatosis: a comprehensive review of safety and efficacy
Orphanet Journal of Rare Diseases (2025)
-
Cerebrotendinous Xanthomatosis patients with late diagnosed in single orthopedic clinic: two novel variants in the CYP27A1 gene
Orphanet Journal of Rare Diseases (2024)
-
A double CYP27A1 gene mutation in spinal cerebrotendinous xanthomatosis in a patient presenting with spastic gait: a case report
Journal of Medical Case Reports (2024)